The RNA-binding landscapes of two SR proteins reveal unique functions and binding to diverse RNA classes
- PMID: 22436691
- PMCID: PMC3439968
- DOI: 10.1186/gb-2012-13-3-r17
The RNA-binding landscapes of two SR proteins reveal unique functions and binding to diverse RNA classes
Abstract
Background: The SR proteins comprise a family of essential, structurally related RNA binding proteins. The complexity of their RNA targets and specificity of RNA recognition in vivo is not well understood. Here we use iCLIP to globally analyze and compare the RNA binding properties of two SR proteins, SRSF3 and SRSF4, in murine cells.
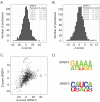
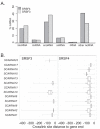
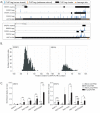
Results: SRSF3 and SRSF4 binding sites mapped to largely non-overlapping target genes, and in vivo consensus binding motifs were distinct. Interactions with intronless and intron-containing mRNAs as well as non-coding RNAs were detected. Surprisingly, both SR proteins bound to the 3' ends of the majority of intronless histone transcripts, implicating SRSF3 and SRSF4 in histone mRNA metabolism. In contrast, SRSF3 but not SRSF4 specifically bound transcripts encoding numerous RNA binding proteins. Remarkably, SRSF3 was shown to modulate alternative splicing of its own as well as three other transcripts encoding SR proteins. These SRSF3-mediated splicing events led to downregulation of heterologous SR proteins via nonsense-mediated decay.
Conclusions: SRSF3 and SRSF4 display unique RNA binding properties underlying diverse cellular regulatory mechanisms, with shared as well as unique coding and non-coding targets. Importantly, CLIP analysis led to the discovery that SRSF3 cross-regulates the expression of other SR protein family members.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

