Serotonin 5-HT2C receptor-mediated inhibition of the M-current in hypothalamic POMC neurons
- PMID: 22436698
- PMCID: PMC3378066
- DOI: 10.1152/ajpendo.00565.2011
Serotonin 5-HT2C receptor-mediated inhibition of the M-current in hypothalamic POMC neurons
Abstract
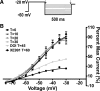
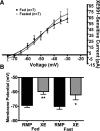
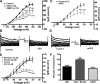
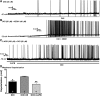
Hypothalamic proopiomelanocortin (POMC) neurons are controlled by many central signals, including serotonin. Serotonin increases POMC activity and reduces feeding behavior via serotonion [5-hydroxytryptamine (5-HT)] receptors by modulating K(+) currents. A potential K(+) current is the M-current, a noninactivating, subthreshold outward K(+) current. Previously, we found that M-current activity was highly reduced in fasted vs. fed states in neuropeptide Y neurons. Because POMC neurons also respond to energy states, we hypothesized that fasting may alter the M-current and/or its modulation by serotonergic input to POMC neurons. Using visualized-patch recording in neurons from fed male enhanced green fluorescent protein-POMC transgenic mice, we established that POMC neurons expressed a robust M-current (102.1 ± 6.7 pA) that was antagonized by the selective KCNQ channel blocker XE-991 (40 μM). However, the XE-991-sensitive current in POMC neurons did not differ between fed and fasted states. To determine if serotonin suppresses the M-current via the 5-HT(2C) receptor, we examined the effects of the 5-HT(2A)/5-HT(2C) receptor agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) on the M-current. Indeed, DOI attenuated the M-current by 34.5 ± 6.9% and 42.0 ± 5.3% in POMC neurons from fed and fasted male mice, respectively. In addition, the 5-HT(1B)/5-HT(2C) receptor agonist m-chlorophenylpiperazine attenuated the M-current by 42.4 ± 5.4% in POMC neurons from fed male mice. Moreover, the selective 5-HT(2C) receptor antagonist RS-102221 abrogated the actions of DOI in suppressing the M-current. Collectively, these data suggest that although M-current expression does not differ between fed and fasted states in POMC neurons, serotonin inhibits the M-current via activation of 5-HT(2C) receptors to increase POMC neuronal excitability and, subsequently, reduce food intake.
Figures


Similar articles
-
Serotonin 5-hydroxytryptamine2C receptor signaling in hypothalamic proopiomelanocortin neurons: role in energy homeostasis in females.Mol Pharmacol. 2007 Oct;72(4):885-96. doi: 10.1124/mol.107.038083. Epub 2007 Jul 10. Mol Pharmacol. 2007. PMID: 17622577
-
Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels.Neuron. 2011 Aug 11;71(3):488-97. doi: 10.1016/j.neuron.2011.06.012. Neuron. 2011. PMID: 21835345 Free PMC article.
-
Antiallodynic effects of intrathecally administered 5-HT(2C) receptor agonists in rats with nerve injury.Pain. 2004 Mar;108(1-2):163-9. doi: 10.1016/j.pain.2003.12.019. Pain. 2004. PMID: 15109520
-
Interaction of 5-HT2A and 5-HT2C receptors in R(-)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice.J Pharmacol Exp Ther. 2010 Dec;335(3):728-34. doi: 10.1124/jpet.110.172247. Epub 2010 Sep 21. J Pharmacol Exp Ther. 2010. PMID: 20858706 Free PMC article.
-
Sex differences in the cannabinoid regulation of energy homeostasis.Psychoneuroendocrinology. 2009 Dec;34 Suppl 1(0 1):S237-46. doi: 10.1016/j.psyneuen.2009.04.007. Psychoneuroendocrinology. 2009. PMID: 19427130 Free PMC article. Review.
Cited by
-
Organophosphate Flame-Retardants Alter Adult Mouse Homeostasis and Gene Expression in a Sex-Dependent Manner Potentially Through Interactions With ERα.Toxicol Sci. 2018 Mar 1;162(1):212-224. doi: 10.1093/toxsci/kfx238. Toxicol Sci. 2018. PMID: 29112739 Free PMC article.
-
Differential gene regulation of GHSR signaling pathway in the arcuate nucleus and NPY neurons by fasting, diet-induced obesity, and 17β-estradiol.Mol Cell Endocrinol. 2016 Feb 15;422:42-56. doi: 10.1016/j.mce.2015.11.007. Epub 2015 Nov 11. Mol Cell Endocrinol. 2016. PMID: 26577678 Free PMC article.
-
Ethanol induced adaptations in 5-HT2c receptor signaling in the bed nucleus of the stria terminalis: implications for anxiety during ethanol withdrawal.Neuropharmacology. 2015 Feb;89:157-67. doi: 10.1016/j.neuropharm.2014.09.003. Epub 2014 Sep 16. Neuropharmacology. 2015. PMID: 25229718 Free PMC article.
-
5-HT2C Receptor Stimulation in Obesity Treatment: Orthosteric Agonists vs. Allosteric Modulators.Nutrients. 2023 Mar 17;15(6):1449. doi: 10.3390/nu15061449. Nutrients. 2023. PMID: 36986191 Free PMC article. Review.
-
Ion channels in the central regulation of energy and glucose homeostasis.Front Neurosci. 2013 May 23;7:85. doi: 10.3389/fnins.2013.00085. eCollection 2013. Front Neurosci. 2013. PMID: 23734095 Free PMC article.
References
-
- Bickerdike MJ. 5-HT2C receptor agonists as potential drugs for the treatment of obesity. Curr Top Med Chem 3: 885–897, 2003 - PubMed
-
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, Fontana DJ, Trinh J, Rocha CL, Dawson MW, Flippin LA, Eglen RM. RS-102221: a novel high affinity and selective, 5-HT2c receptor antagonist. Neuropharmacology 36: 621–629, 1997 - PubMed
-
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005 - PubMed
-
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 6: 850–862, 2005 - PubMed
-
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22: 221–232, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

