MicroRNA-34a upregulation during seizure-induced neuronal death
- PMID: 22436728
- PMCID: PMC3317348
- DOI: 10.1038/cddis.2012.23
MicroRNA-34a upregulation during seizure-induced neuronal death
Abstract
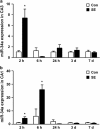
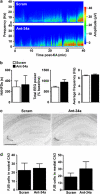
MicroRNAs (miRNAs) are short, noncoding RNAs that function as posttranscriptional regulators of gene expression by controlling translation of mRNAs. A subset of miRNAs may be critical for the control of cell death, including the p53-regulated miRNA, miR-34a. Because seizures activate p53, and p53-deficient mice are reportedly resistant to damage caused by prolonged seizures, we investigated the role of miR-34a in seizure-induced neuronal death in vivo. Status epilepticus was induced by intra-amygdala microinjection of kainic acid in mice. This led to an early (2 h) multifold upregulation of miR-34a in the CA3 and CA1 hippocampal subfields and lower protein levels of mitogen-activated kinase kinase kinase 9, a validated miR-34a target. Immunoprecipitation of the RNA-induced silencing complex component, Argonaute-2, eluted significantly higher levels of miR-34a after seizures. Injection of mice with pifithrin-α, a putative p53 inhibitor, prevented miR-34a upregulation after seizures. Intracerebroventricular injection of antagomirs targeting miR-34a reduced hippocampal miR-34a levels and had a small modulatory effect on apoptosis-associated signaling, but did not prevent hippocampal neuronal death in models of either severe or moderate severity status epilepticus. Thus, prolonged seizures cause subfield-specific, temporally restricted upregulation of miR-34a, which may be p53 dependent, but miR-34a is probably not important for seizure-induced neuronal death in this model.
Figures




References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–139. - PubMed
-
- Peters L, Meister G. Argonaute proteins: mediators of RNA silencing. Mol Cell. 2007;26:611–623. - PubMed
-
- Aronica E, Fluiter K, Iyer A, Zurolo E, Vreijling J, van Vliet EA, et al. Expression pattern of miR-146a, an inflammation-associated microRNA, in experimental and human temporal lobe epilepsy. Eur J Neurosci. 2010;31:1100–1107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

