Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study
- PMID: 22436847
- PMCID: PMC3353803
- DOI: 10.1093/cvr/cvs126
Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study
Abstract
Aims: Vascular cartilaginous metaplasia and calcification are common in patients with atherosclerosis. However, sources of cells contributing to the development of this complication are currently unknown. In this study, we ascertained the origin of cells that give rise to cartilaginous and bony elements in atherosclerotic vessels.
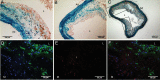
Methods and results: We utilized genetic fate mapping strategies to trace cells of smooth muscle (SM) origin via SM22α-Cre recombinase and Rosa26-LacZ Cre reporter alleles. In animals expressing both transgenes, co-existence within a single cell of β-galactosidase [marking cells originally derived from SM cells (SMCs)] with osteochondrogenic (Runx2/Cbfa1) or chondrocytic (Sox9, type II collagen) markers, along with simultaneous loss of SM lineage proteins, provides a strong evidence supporting reprogramming of SMCs towards osteochondrogenic or chondrocytic differentiation. Using this technique, we found that vascular SMCs accounted for ~80% of Runx2/Cbfa1-positive cells and almost all of type II collagen-positive cells (~98%) in atherosclerotic vessels of LDLr-/- and ApoE-/- mice. We also assessed contribution from bone marrow (BM)-derived cells via analysing vessels dissected from chimerical ApoE-/- mice transplanted with green fluorescence protein-expressing BM. Marrow-derived cells were found to account for ~20% of Runx2/Cbfa1-positive cells in calcified atherosclerotic vessels of ApoE-/- mice.
Conclusion: Our results are the first to definitively identify cell sources attributable to atherosclerotic intimal calcification. SMCs were found to be a major contributor that reprogrammed its lineage towards osteochondrogenesis. Marrow-derived cells from the circulation also contributed significantly to the early osteochondrogenic differentiation in atherosclerotic vessels.
Figures





References
-
- Taylor AJ, Burke AP, O'Malley PG, Farb A, Malcom GT, Smialek J, et al. A comparison of the Framingham risk index, coronary artery calcification, and culprit plaque morphology in sudden cardiac death. Circulation. 2000;101:1243–1248. - PubMed
-
- Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31:16–23. - PubMed
-
- Mizobuchi M, Towler D, Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. J Am Soc Nephrol. 2009;20:1453–1464. - PubMed
-
- Ehara S, Kobayashi Y, Yoshiyama M, Shimada K, Shimada Y, Fukuda D, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–3429. - PubMed
-
- Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg's sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

