Caspase activation contributes to astrogliosis
- PMID: 22436850
- PMCID: PMC3319728
- DOI: 10.1016/j.brainres.2012.02.056
Caspase activation contributes to astrogliosis
Abstract
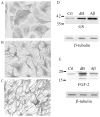
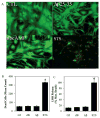
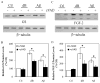
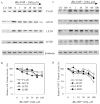
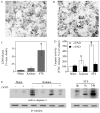
Caspases, a family of cysteine proteases, are widely activated in neurons and glia in the injured brain, a response thought to induce apoptosis. However, caspase activation in astrocytes following injury is not strongly associated with apoptosis. The present study investigates the potential role of caspase activation in astrocytes with another characteristic response to neural injury, astrogliosis. Caspase activity and morphological and biochemical indices of astrogliosis and apoptosis were assessed in (i) cultured neonatal rat astrocytes treated with astrogliosis-inducing stimuli (dibutryl cAMP, β-amyloid peptide), and (ii) cultures of adult rat hippocampal astrocytes generated from control and kainate-lesioned rats. The effects of broad spectrum and specific pharmacological caspase inhibitors were assessed on indicators of astrogliosis, including stellate morphology and expression of glutamine synthetase and fibroblast growth factor-2. Reactive neonatal and adult astrocytes demonstrated an increase in total caspase activity with a corresponding increase in the expression of active caspase-3 in the absence of cell death. Broad spectrum caspase inhibition with zVAD significantly attenuated increases in glutamine synthetase and fibroblast growth factor-2 in the reactive astrocytes. In the reactive neonatal astrocyte cultures, specific inhibition of caspases-3 and -11 also attenuated glutamine synthetase and fibroblast growth factor-2 expression, but did not reverse the morphological reactive phenotype. Astrogliosis is observed in all forms of brain injury and despite extensive study, its molecular triggers remain largely unknown. While previous studies have demonstrated active caspases in astrocytes following acute brain injury, here we present evidence functionally implicating the caspases in astrogliosis.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
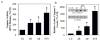
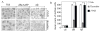



References
-
- Abe K, Kato M, Saito H. Human amylin mimicks amyloid [beta] protein-induced reactive gliosis and inhibition of cellular redox activity in cultured astrocytes. Brain Res. 1997;762:285–288. - PubMed
-
- Abe K, Saito H. Na+ and K+ dependence of morphological changes of cultured rat cortical astrocytes. Pharmacol Toxicol. 2001;88:319–24. - PubMed
-
- Abe K, Saito H. The p44/42 mitogen-activated protein kinase cascade is involved in the induction and maintenance of astrocyte stellation mediated by protein kinase C. Neurosci Res. 2000;36:251–7. - PubMed
-
- Acarin L, Villapol S, Faiz M, Rohn TT, Castellano B, González B. Caspase-3 activation in astrocytes following postnatal excitotoxic damage correlates with cytoskeletal remodeling but not with cell death or proliferation. Glia. 2007;55:954–965. - PubMed
-
- Araujo DM, Cotman CW. [beta]-Amyloid stimulates glial cells in vitro to produce growth factors that accumulate in senile plaques in Alzheimer’s disease. Brain Res. 1992;569:141–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

