Dentin-cement interfacial interaction: calcium silicates and polyalkenoates
- PMID: 22436906
- PMCID: PMC4077527
- DOI: 10.1177/0022034512443068
Dentin-cement interfacial interaction: calcium silicates and polyalkenoates
Abstract
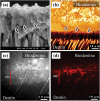
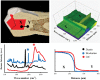
The interfacial properties of a new calcium-silicate-based coronal restorative material (Biodentine™) and a glass-ionomer cement (GIC) with dentin have been studied by confocal laser scanning microscopy (CLSM), scanning electron microscopy (SEM), micro-Raman spectroscopy, and two-photon auto-fluorescence and second-harmonic-generation (SHG) imaging. Results indicate the formation of tag-like structures alongside an interfacial layer called the "mineral infiltration zone", where the alkaline caustic effect of the calcium silicate cement's hydration products degrades the collagenous component of the interfacial dentin. This degradation leads to the formation of a porous structure which facilitates the permeation of high concentrations of Ca(2+), OH(-), and CO(3) (2-) ions, leading to increased mineralization in this region. Comparison of the dentin-restorative interfaces shows that there is a dentin-mineral infiltration with the Biodentine, whereas polyacrylic and tartaric acids and their salts characterize the penetration of the GIC. A new type of interfacial interaction, "the mineral infiltration zone", is suggested for these calcium-silicate-based cements.
Conflict of interest statement
The author(s) declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures


References
-
- Andersen M, Lund A, Andreasen JO, Andreasen FM. (1992). In vitro solubility of human pulp tissue in calcium hydroxide and sodium hypochlorite. Dent Traumatol 8:104-108 - PubMed
-
- Andreasen JO, Farik B, Munksgaard EC. (2002). Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol 18:134-137 - PubMed
-
- Camilleri J, Montesin FE, Papaioannou S, McDonald F, Pitt Ford TR. (2004). Biocompatibility of two commercial forms of mineral trioxide aggregate. Int Endod J 37:699-704 - PubMed
-
- Camilleri J, Cutajar A, Mallia B. (2011). Hydration characteristics of zirconium oxide replaced Portland cement for use as a root-end filling material. Dent Mater 27:845-854 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

