Structure of the human κ-opioid receptor in complex with JDTic
- PMID: 22437504
- PMCID: PMC3356457
- DOI: 10.1038/nature10939
Structure of the human κ-opioid receptor in complex with JDTic
Abstract
Opioid receptors mediate the actions of endogenous and exogenous opioids on many physiological processes, including the regulation of pain, respiratory drive, mood, and--in the case of κ-opioid receptor (κ-OR)--dysphoria and psychotomimesis. Here we report the crystal structure of the human κ-OR in complex with the selective antagonist JDTic, arranged in parallel dimers, at 2.9 Å resolution. The structure reveals important features of the ligand-binding pocket that contribute to the high affinity and subtype selectivity of JDTic for the human κ-OR. Modelling of other important κ-OR-selective ligands, including the morphinan-derived antagonists norbinaltorphimine and 5'-guanidinonaltrindole, and the diterpene agonist salvinorin A analogue RB-64, reveals both common and distinct features for binding these diverse chemotypes. Analysis of site-directed mutagenesis and ligand structure-activity relationships confirms the interactions observed in the crystal structure, thereby providing a molecular explanation for κ-OR subtype selectivity, and essential insights for the design of compounds with new pharmacological properties targeting the human κ-OR.
Figures


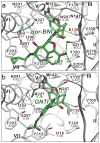

Comment in
-
Structural biology: How opioid drugs bind to receptors.Nature. 2012 May 16;485(7398):314-7. doi: 10.1038/485314a. Nature. 2012. PMID: 22596150 Free PMC article.
References
-
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. - PubMed
-
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

