Intraoperative detection and removal of microscopic residual sarcoma using wide-field imaging
- PMID: 22437667
- PMCID: PMC3532657
- DOI: 10.1002/cncr.27458
Intraoperative detection and removal of microscopic residual sarcoma using wide-field imaging
Abstract
Background: The goal of limb-sparing surgery for a soft tissue sarcoma of the extremity is to remove all malignant cells while preserving limb function. After initial surgery, microscopic residual disease in the tumor bed will cause a local recurrence in approximately 33% of patients with sarcoma. To help identify these patients, the authors developed an in vivo imaging system to investigate the suitability of molecular imaging for intraoperative visualization.
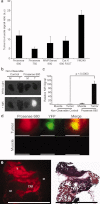
Methods: A primary mouse model of soft tissue sarcoma and a wide field-of-view imaging device were used to investigate a series of exogenously administered, near-infrared (NIR) fluorescent probes activated by cathepsin proteases for real-time intraoperative imaging.
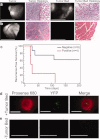
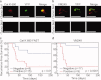
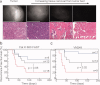
Results: The authors demonstrated that exogenously administered cathepsin-activated probes can be used for image-guided surgery to identify microscopic residual NIR fluorescence in the tumor beds of mice. The presence of residual NIR fluorescence was correlated with microscopic residual sarcoma and local recurrence. The removal of residual NIR fluorescence improved local control.
Conclusions: The authors concluded that their technique has the potential to be used for intraoperative image-guided surgery to identify microscopic residual disease in patients with cancer.
Copyright © 2012 American Cancer Society.
Figures

References
-
- Lewis JJ, Leung D, Casper ES, Woodruff J, Hajdu SI, Brennan MF. Multifactorial analysis of long-term follow-up (more than 5 years) of primary extremity sarcoma. Arch Surg. 1999;134:190–194. - PubMed
-
- Sabolch A, Feng M, Griffith K, et al. Risk factors for local recurrence and metastasis in soft tissue sarcomas of the extremity [published online ahead of print February 17, 2011] Am J Clin Oncol. 2011 - PubMed
-
- Sadoski C, Suit HD, Rosenberg A, Mankin H, Efird J. Preoperative radiation, surgical margins, and local control of extremity sarcomas of soft tissues. J Surg Oncol. 1993;52:223–230. - PubMed
-
- Allweis TM, Kaufman Z, Lelcuk S, et al. A prospective, randomized, controlled, multicenter study of a real-time, intraoperative probe for positive margin detection in breast-conserving surgery. Am J Surg. 2008;196:483–489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

