Brain activity during sympathetic response in anticipation and experience of pain
- PMID: 22438199
- PMCID: PMC6870103
- DOI: 10.1002/hbm.22035
Brain activity during sympathetic response in anticipation and experience of pain
Abstract
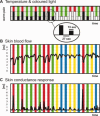
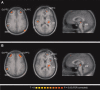
Pain is a multidimensional phenomenon with sensory, affective, and autonomic components. Here, we used parametric functional magnetic resonance imaging (fMRI) to correlate regional brain activity with autonomic responses to (i) painful stimuli and to (ii) anticipation of pain. The autonomic parameters used for correlation were (i) skin blood flow (SBF) and (ii) skin conductance response (SCR). During (i) experience of pain and (ii) anticipation of pain, activity in the insular cortex, anterior cingulate cortex (ACC), prefrontal cortex (PFC), posterior parietal cortex (PPC), secondary somatosensory cortex (S2), thalamus, and midbrain correlated with sympathetic outflow. A conjunction analysis revealed a common central sympathetic network for (i) pain experience and (ii) pain anticipation with similar correlations between brain activity and sympathetic parameters in the anterior insula, prefrontal cortex, thalamus, midbrain, and temporoparietal junction. Therefore, we here describe shared central neural networks involved in the central autonomic processing of the experience and anticipation of pain.
Keywords: autonomic nervous system; fMRI; neuropathic pain; pain; somatosensory system.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


References
-
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK ( 2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484. - PubMed
-
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I ( 2002): Imaging how attention modulates pain in humans using functional MRI. Brain 125( Part 2): 310–319. - PubMed
-
- Bechara A, Tranel D, Damasio H ( 2000): Characterization of the decision‐making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123( Part 11): 2189–2202. - PubMed
-
- Benarroch EE ( 2006): Pain‐autonomic interactions. Neurol Sci 27( Suppl 2): S130–S133. - PubMed
-
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C ( 2006): Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120: 8–15. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

