Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells
- PMID: 22438251
- PMCID: PMC3362361
- DOI: 10.1182/blood-2011-11-392324
Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells
Erratum in
- Blood. 2012 Nov 22;120(22):4447
Abstract
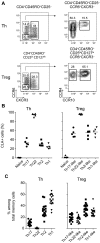
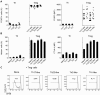
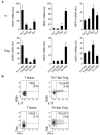
FOXP3+ regulatory T (Treg) cells are a broadly acting and potent anti-inflammatory population of CD4+ T cells essential for maintaining immune homeostasis and preventing debilitating autoimmunity. Based on chemokine receptor expression, we identified distinct populations of Treg cells in human blood expected to colocalize with different Th cell subsets. Although each population was functionally suppressive, they displayed unique patterns of pro- and anti-inflammatory cytokine production, differentially expressed lineage-specifying transcription factors, and responded differently to antigens associated with Th1 and Th17 responses. These results highlight a previously unappreciated degree of phenotypic and functional diversity in human Treg cells that allows subsets with unique specificities and immunomodulatory functions to be targeted to defined immune environments during different types of inflammatory responses.
Figures


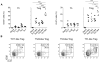

References
-
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10(8):857–863. - PubMed
-
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10(8):864–871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

