Demyelinating diseases: myeloperoxidase as an imaging biomarker and therapeutic target
- PMID: 22438365
- PMCID: PMC3329272
- DOI: 10.1148/radiol.12111593
Demyelinating diseases: myeloperoxidase as an imaging biomarker and therapeutic target
Abstract
Purpose: To evaluate myeloperoxidase (MPO) as a newer therapeutic target and bis-5-hydroxytryptamide-diethylenetriaminepentaacetate-gadolinium (Gd) (MPO-Gd) as an imaging biomarker for demyelinating diseases such as multiple sclerosis (MS) by using experimental autoimmune encephalomyelitis (EAE), a murine model of MS.
Materials and methods: Animal experiments were approved by the institutional animal care committee. EAE was induced in SJL mice by using proteolipid protein (PLP), and mice were treated with either 4-aminobenzoic acid hydrazide (ABAH), 40 mg/kg injected intraperitoneally, an irreversible inhibitor of MPO, or saline as control, and followed up to day 40 after induction. In another group of SJL mice, induction was performed without PLP as shams. The mice were imaged by using MPO-Gd to track changes in MPO activity noninvasively. Imaging results were corroborated by enzymatic assays, flow cytometry, and histopathologic analyses. Significance was computed by using the t test or Mann-Whitney U test.
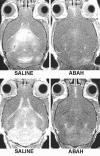
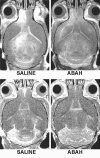
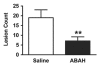
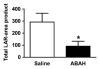
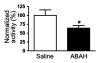
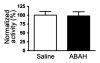
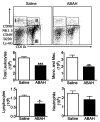
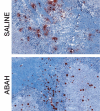
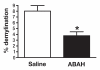
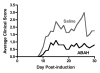
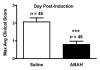
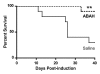
Results: There was a 2.5-fold increase in myeloid cell infiltration in the brain (P = .026), with a concomitant increase in brain MPO level (P = .0087). Inhibiting MPO activity with ABAH resulted in decrease in MPO-Gd-positive lesion volume (P = .012), number (P = .009), and enhancement intensity (P = .03) at MR imaging, reflecting lower local MPO activity (P = .03), compared with controls. MPO inhibition was accompanied by decreased demyelination (P = .01) and lower inflammatory cell recruitment in the brain (P < .0001), suggesting a central MPO role in inflammatory demyelination. Clinically, MPO inhibition significantly reduced the severity of clinical symptoms (P = .0001) and improved survival (P = .0051) in mice with EAE.
Conclusion: MPO may be a key mediator of myeloid inflammation and tissue damage in EAE. Therefore, MPO could represent a promising therapeutic target, as well as an imaging biomarker, for demyelinating diseases and potentially for other diseases in which MPO is implicated.
Figures






References
-
- Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci Lett 2008;444(2):195–198 - PubMed
-
- Heppner FL, Greter M, Marino D, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 2005;11(2):146–152 - PubMed
-
- Bartnik BL, Juurlink BH, Devon RM. Macrophages: their myelinotrophic or neurotoxic actions depend upon tissue oxidative stress. Mult Scler 2000;6(1):37–42 - PubMed
-
- Jack C, Ruffini F, Bar-Or A, Antel JP. Microglia and multiple sclerosis. J Neurosci Res 2005;81(3):363–373 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

