Formulating spatially varying performance in the statistical fusion framework
- PMID: 22438513
- PMCID: PMC3368083
- DOI: 10.1109/TMI.2012.2190992
Formulating spatially varying performance in the statistical fusion framework
Erratum in
- IEEE Trans Med Imaging. 2012 Jul;31(7):1505
Abstract
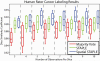
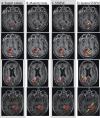
To date, label fusion methods have primarily relied either on global [e.g., simultaneous truth and performance level estimation (STAPLE), globally weighted vote] or voxelwise (e.g., locally weighted vote) performance models. Optimality of the statistical fusion framework hinges upon the validity of the stochastic model of how a rater errs (i.e., the labeling process model). Hitherto, approaches have tended to focus on the extremes of potential models. Herein, we propose an extension to the STAPLE approach to seamlessly account for spatially varying performance by extending the performance level parameters to account for a smooth, voxelwise performance level field that is unique to each rater. This approach, Spatial STAPLE, provides significant improvements over state-of-the-art label fusion algorithms in both simulated and empirical data sets.
Figures







References
-
- Crespo-Facorro B, Kim J, Andreasen N, O'Leary D, Wiser A, Bailey J, Harris G, Magnotta V. Human frontal cortex: an MRI-based parcellation method. NeuroImage. 1999;10:500–519. - PubMed
-
- Tsang O, Gholipour A, Kehtarnavaz N, Gopinath K, Briggs R, Panahi I. Comparison of tissue segmentation algorithms in neuroimage analysis software tools. Conference proceedings : ... Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 20082008:3924–8. - PubMed
-
- Filippi M, Horsfield M, Bressi S, Martinelli V, Baratti C, Reganati P, Campi A, Miller D, Comi G. Intra-and inter-observer agreement of brain MRI lesion volume measurements in multiple sclerosis. Brain. 1995;118:1593. - PubMed
-
- Ashton E, Takahashi C, Berg M, Goodman A, Totterman S, Ekholm S. Accuracy and reproducibility of manual and semiautomated quantification of MS lesions by MRI. Journal of Magnetic Resonance Imaging. 2003;17:300–308. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

