Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking
- PMID: 22438585
- PMCID: PMC3350547
- DOI: 10.1091/mbc.E11-12-1006
Reggies/flotillins regulate E-cadherin-mediated cell contact formation by affecting EGFR trafficking
Abstract
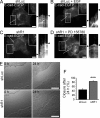
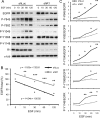
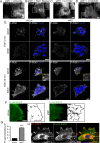
The reggie/flotillin proteins are implicated in membrane trafficking and, together with the cellular prion protein (PrP), in the recruitment of E-cadherin to cell contact sites. Here, we demonstrate that reggies, as well as PrP down-regulation, in epithelial A431 cells cause overlapping processes and abnormal formation of adherens junctions (AJs). This defect in cell adhesion results from reggie effects on Src tyrosine kinases and epidermal growth factor receptor (EGFR): loss of reggies reduces Src activation and EGFR phosphorylation at residues targeted by Src and c-cbl and leads to increased surface exposure of EGFR by blocking its internalization. The prolonged EGFR signaling at the plasma membrane enhances cell motility and macropinocytosis, by which junction-associated E-cadherin is internalized and recycled back to AJs. Accordingly, blockage of EGFR signaling or macropinocytosis in reggie-deficient cells restores normal AJ formation. Thus, by promoting EGFR internalization, reggies restrict the EGFR signaling involved in E-cadherin macropinocytosis and recycling and regulate AJ formation and dynamics and thereby cell adhesion.
Figures






References
-
- Araki N, Egami Y, Watanabe Y, Hatae T. Phosphoinositide metabolism during membrane ruffling and macropinosome formation in EGF-stimulated A431 cells. Exp Cell Res. 2007;313:1496–1507. - PubMed
-
- Bryant DM, Kerr MC, Hammond LA, Joseph SR, Mostov KE, Teasdale RD, Stow JL. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J Cell Sci. 2007;120:1818–1828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

