Post-translational modifications of nuclear receptors and human disease
- PMID: 22438791
- PMCID: PMC3309075
- DOI: 10.1621/nrs.10001
Post-translational modifications of nuclear receptors and human disease
Abstract
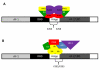
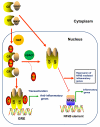
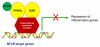
Nuclear receptors (NR) impact a myriad of physiological processes including homeostasis, reproduction, development, and metabolism. NRs are regulated by post-translational modifications (PTM) that markedly impact receptor function. Recent studies have identified NR PTMs that are involved in the onset and progression of human diseases, including cancer. The majority of evidence linking NR PTMs with disease has been demonstrated for phosphorylation, acetylation and sumoylation of androgen receptor (AR), estrogen receptor α (ERα), glucocorticoid receptor (GR) and peroxisome proliferator activated receptor γ (PPARγ). Phosphorylation of AR has been associated with hormone refractory prostate cancer and decreased disease-specific survival. AR acetylation and sumoylation increased growth of prostate cancer tumor models. AR phosphorylation reduced the toxicity of the expanded polyglutamine AR in Kennedy's Disease as a consequence of reduced ligand binding. A comprehensive evaluation of ERα phosphorylation in breast cancer revealed several sites associated with better clinical outcome to tamoxifen therapy, whereas other phosphorylation sites were associated with poorer clinical outcome. ERα acetylation and sumoylation may also have predictive value for breast cancer. GR phosphorylation and acetylation impact GR responsiveness to glucocorticoids that are used as anti-inflammatory drugs. PPARγ phosphorylation can regulate the balance between growth and differentiation in adipose tissue that is linked to obesity and insulin resistance. Sumoylation of PPARγ is linked to repression of inflammatory genes important in patients with inflammatory diseases. NR PTMs provide an additional measure of NR function that can be used as both biomarkers of disease progression, and predictive markers for patient response to NR-directed treatments.
Figures




References
-
- Abbinante-Nissen J. M., Simpson L. G., Leikauf G. D. Corticosteroids increase secretory leukocyte protease inhibitor transcript levels in airway epithelial cells. Am J Physiol. 1995;268:L601–6. - PubMed
-
- Adcock I. M., Ito K. Molecular mechanisms of corticosteroid actions. Monaldi Arch Chest Dis. 2000;55:256–66. - PubMed
-
- Al-Ramahi I., Lam Y. C., Chen H. K., de Gouyon B., Zhang M., Perez A. M., Branco J., de Haro M., Patterson C., Zoghbi H. Y., Botas J. CHIP protects from the neurotoxicity of expanded and wild-type ataxin-1 and promotes their ubiquitination and degradation. J Biol Chem. 2006;281:26714–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

