Retinoic acid synthesis and functions in early embryonic development
- PMID: 22439772
- PMCID: PMC3325842
- DOI: 10.1186/2045-3701-2-11
Retinoic acid synthesis and functions in early embryonic development
Abstract
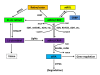
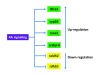
Retinoic acid (RA) is a morphogen derived from retinol (vitamin A) that plays important roles in cell growth, differentiation, and organogenesis. The production of RA from retinol requires two consecutive enzymatic reactions catalyzed by different sets of dehydrogenases. The retinol is first oxidized into retinal, which is then oxidized into RA. The RA interacts with retinoic acid receptor (RAR) and retinoic acid X receptor (RXR) which then regulate the target gene expression. In this review, we have discussed the metabolism of RA and the important components of RA signaling pathway, and highlighted current understanding of the functions of RA during early embryonic development.
Figures



References
-
- Duriancik DM, Lackey DE, Hoag KA. Vitamin A as a regulator of antigen presenting cells. J Nutr. pp. 1395–1399. - PubMed
LinkOut - more resources
Full Text Sources

