The R-spondin protein family
- PMID: 22439850
- PMCID: PMC3439965
- DOI: 10.1186/gb-2012-13-3-242
The R-spondin protein family
Abstract
The four vertebrate R-spondin proteins are secreted agonists of the canonical Wnt/β-catenin signaling pathway. These proteins are approximately 35 kDa, and are characterized by two amino-terminal furin-like repeats, which are necessary and sufficient for Wnt signal potentiation, and a thrombospondin domain situated more towards the carboxyl terminus that can bind matrix glycosaminoglycans and/or proteoglycans. Although R-spondins are unable to initiate Wnt signaling, they can potently enhance responses to low-dose Wnt proteins. In humans, rare disruptions of the gene encoding R-spondin1 cause a syndrome of XX sex reversal (phenotypic male), palmoplantar keratosis (a thickening of the palms and soles caused by excess keratin formation) and predisposition to squamous cell carcinoma of the skin. Mutations in the gene encoding R-spondin4 cause anonychia (absence or hypoplasia of nails on fingers and toes). Recently, leucine-rich repeat-containing G-protein-coupled receptor (Lgr)4, Lgr5 and Lgr6, three closely related orphans of the leucine-rich repeat family of G-protein-coupled receptors, have been identified as receptors for R-spondins. Lgr5 and Lgr6 are markers for adult stem cells. Because R-spondins are potent stimulators of adult stem cell proliferation in vivo and in vitro, these findings might guide the therapeutic use of R-spondins in regenerative medicine.
Figures

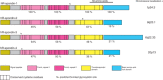
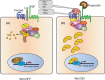

References
-
- Kamata T, Katsube K, Michikawa M, Yamada M, Takada S, Mizusawa H. R-spondin, a novel gene with thrombospondin type 1 domain, was expressed in the dorsal neural tube and affected in Wnts mutants. Biochim Biophys Acta. 2004;1676:51–62. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

