Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells
- PMID: 22439936
- PMCID: PMC3311997
- DOI: 10.1016/j.ccr.2012.01.008
Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells
Abstract
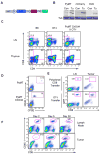
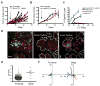
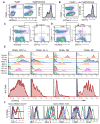
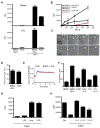
The nature and site of tumor-antigen presentation to immune T cells by bone-marrow-derived cells within the tumor microenvironment remains unresolved. We generated a fluorescent mouse model of spontaneous immunoevasive breast cancer and identified a subset of myeloid cells with significant similarity to dendritic cells and macrophages that constitutively ingest tumor-derived proteins and present processed tumor antigens to reactive T cells. Using intravital live imaging, we determined that infiltrating tumor-specific T cells engage in long-lived interactions with these cells, proximal to the tumor. In vitro, these cells capture cytotoxic T cells in signaling-competent conjugates but do not support full activation or sustain cytolysis. The spatiotemporal dynamics revealed here implicate nonproductive interactions between T cells and antigen-presenting cells on the tumor margin.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Ambe K, Mori M, Enjoji M. S-100 protein-positive dendritic cells in colorectal adenocarcinomas. Distribution and relation to the clinical prognosis. Cancer. 1989;63:496–503. - PubMed
-
- Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. Journal of Immunology. 2007;178:1268–76. - PubMed
-
- Bruggen P van der, Traversari C, Chomez P, Lurquin C, Plaen E De, Eynde B Van den, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

