Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury
- PMID: 22439962
- PMCID: PMC3360508
- DOI: 10.1089/ten.TEA.2011.0614
Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury
Abstract
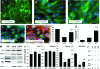
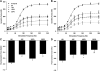
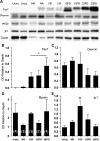
Volumetric muscle loss (VML) can result from trauma and surgery in civilian and military populations, resulting in irrecoverable functional and cosmetic deficits that cannot be effectively treated with current therapies. Previous work evaluated a bioreactor-based tissue engineering approach in which muscle derived cells (MDCs) were seeded onto bladder acellular matrices (BAM) and mechanically preconditioned. This first generation tissue engineered muscle repair (TEMR) construct exhibited a largely differentiated cellular morphology consisting primarily of myotubes, and moreover, significantly improved functional recovery within 2 months of implantation in a murine latissimus dorsi (LD) muscle with a surgically created VML injury. The present report extends these initial observations to further document the importance of the cellular phenotype and composition of the TEMR construct in vitro to the functional recovery observed following implantation in vivo. To this end, three distinct TEMR constructs were created by seeding MDCs onto BAM as follows: (1) a short-term cellular proliferation of MDCs to generate primarily myoblasts without bioreactor preconditioning (TEMR-1SP), (2) a prolonged cellular differentiation and maturation period that included bioreactor preconditioning (TEMR-1SPD; identical to the first generation TEMR construct), and (3) similar treatment as TEMR-1SPD but with a second application of MDCs during bioreactor preconditioning (TEMR-2SPD); simulating aspects of "exercise" in vitro. Assessment of maximal tetanic force generation on retrieved LD muscles in vitro revealed that TEMR-1SP and TEMR-1SPD constructs promoted either an accelerated (i.e., 1 month) or a prolonged (i.e., 2 month postinjury) functional recovery, respectively, of similar magnitude. Meanwhile, TEMR-2SPD constructs promoted both an accelerated and prolonged functional recovery, resulting in twice the magnitude of functional recovery of either TEMR-1SP or TEMR-1SPD constructs. Histological and molecular analyses indicated that TEMR constructs mediated functional recovery via regeneration of functional muscle fibers either at the interface of the construct and the native tissue or within the BAM scaffolding independent of the native tissue. Taken together these findings are encouraging for the further development and clinical application of TEMR constructs as a VML injury treatment.
Figures




References
-
- Carlson B.M. Regeneration fo the completely excised gastrocnemius muscle in the frog and rat from minced muscle fragments. J Morphol. 1968;125:447. - PubMed
-
- Carlson B.M. Faulkner J.A. The regeneration of skeletal muscle fibers following injury: a review. Med Sci Sports Exerc. 1983;15:187. - PubMed
-
- Ciciliot S. Schiaffino S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des. 2010;16:906. - PubMed
-
- White T.P. Devor S.T. Skeletal muscle regeneration and plasticity of grafts. Exerc Sport Sci Rev. 1993;21:263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

