Stem cells in thoracic aortic aneurysms and dissections: potential contributors to aortic repair
- PMID: 22440369
- PMCID: PMC3423336
- DOI: 10.1016/j.athoracsur.2012.01.063
Stem cells in thoracic aortic aneurysms and dissections: potential contributors to aortic repair
Abstract
Background: The hallmark of thoracic aortic aneurysms and dissections (TAAD) is progressive medial degeneration, which can result from excessive tissue destruction and insufficient repair. Although multipotent stem cells (SCs) are important in tissue repair, their role in TAAD is unknown. We sought to determine whether SCs are more abundant in TAAD tissue than in control tissues, and whether SCs within the diseased aortic wall differentiate into functionally relevant cell types.
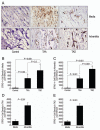
Methods: Using immunohistochemistry, we compared the abundance of STRO-1+ cells, c-kit+ cells, and CD34+ cells in aortic tissue from patients with descending thoracic aortic aneurysms (n=12), patients with chronic descending thoracic aortic dissections (n=18), and age-matched organ donors (n=5). Using double immunofluorescence staining, we evaluated SC differentiation into smooth muscle cells, fibroblasts, and macrophages.
Results: All three cell types were significantly more abundant in the media and adventitia of TAAD tissues than in control tissues. We identified subsets of STRO-1+ cells, c-kit+ cells, and CD34+ cells that also expressed the smooth muscle cell marker SM22-α or fibroblast-specific protein-1, suggesting SC differentiation into smooth muscle cells or fibroblasts. Other STRO-1+ cells expressed the macrophage marker CD68, suggesting differentiation into inflammatory cells.
Conclusions: Stem cells are more abundant in TAAD tissue than in normal aortic tissue. Differentiation of SCs into smooth muscle cells, fibroblasts, and inflammatory cells within the diseased aortic wall suggests that SCs might be involved in both reparative and destructive remodeling processes in TAAD. Understanding the regulation of SC-mediated aortic remodeling will be a critical step toward designing strategies to promote aortic repair and prevent adverse remodeling.
Copyright © 2012 The Society of Thoracic Surgeons. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Notch signaling in descending thoracic aortic aneurysm and dissection.PLoS One. 2012;7(12):e52833. doi: 10.1371/journal.pone.0052833. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300792 Free PMC article.
-
Proinflammatory role of stem cells in abdominal aortic aneurysms.J Vasc Surg. 2015 Nov;62(5):1303-11.e4. doi: 10.1016/j.jvs.2014.04.067. Epub 2014 Jul 3. J Vasc Surg. 2015. PMID: 24997808
-
Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections.J Thorac Cardiovasc Surg. 2006 Mar;131(3):671-8. doi: 10.1016/j.jtcvs.2005.09.018. J Thorac Cardiovasc Surg. 2006. PMID: 16515922
-
From genetics to response to injury: vascular smooth muscle cells in aneurysms and dissections of the ascending aorta.Cardiovasc Res. 2018 Mar 15;114(4):578-589. doi: 10.1093/cvr/cvy006. Cardiovasc Res. 2018. PMID: 29360940 Free PMC article. Review.
-
Advancements in the study of T lymphocytes in thoracic aortic aneurysm and aortic dissection.Tissue Cell. 2025 Apr;93:102768. doi: 10.1016/j.tice.2025.102768. Epub 2025 Jan 30. Tissue Cell. 2025. PMID: 39923647 Review.
Cited by
-
Stem Cell-Derived Exosomes, Autophagy, Extracellular Matrix Turnover, and miRNAs in Cardiac Regeneration during Stem Cell Therapy.Stem Cell Rev Rep. 2017 Feb;13(1):79-91. doi: 10.1007/s12015-016-9696-y. Stem Cell Rev Rep. 2017. PMID: 27807762 Free PMC article. Review.
-
Oxidized phospholipids stimulate production of stem cell factor via NRF2-dependent mechanisms.Angiogenesis. 2018 May;21(2):229-236. doi: 10.1007/s10456-017-9590-5. Epub 2018 Jan 12. Angiogenesis. 2018. PMID: 29330760 Free PMC article.
-
Molecular pathogenesis of genetic and sporadic aortic aneurysms and dissections.Curr Probl Surg. 2017 Mar;54(3):95-155. doi: 10.1067/j.cpsurg.2017.01.001. Epub 2017 Feb 3. Curr Probl Surg. 2017. PMID: 28521856 Free PMC article. Review. No abstract available.
-
Activation of Bone Marrow-Derived Cells and Resident Aortic Cells During Aortic Injury.J Surg Res. 2020 Jan;245:1-12. doi: 10.1016/j.jss.2019.07.013. Epub 2019 Aug 5. J Surg Res. 2020. PMID: 31394402 Free PMC article.
-
Macrophage in Sporadic Thoracic Aortic Aneurysm and Dissection: Potential Therapeutic and Preventing Target.Rev Cardiovasc Med. 2023 Nov 30;24(12):340. doi: 10.31083/j.rcm2412340. eCollection 2023 Dec. Rev Cardiovasc Med. 2023. PMID: 39077089 Free PMC article. Review.
References
-
- Kung HC, Hoyert DL, Xu J, Murphy SL. Deaths: final data for 2005. Natl Vital Stat Rep. 2008;56:1–120. - PubMed
-
- Schlatmann TJ, Becker AE. Pathogenesis of dissecting aneurysm of aorta. Comparative histopathologic study of significance of medial changes. Am J Cardiol. 1977;39:21–6. - PubMed
-
- Dentelli P, Rosso A, Balsamo A, et al. C-KIT, by interacting with the membrane-bound ligand, recruits endothelial progenitor cells to inflamed endothelium. Blood. 2007;109:4264–71. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

