Inducible podocyte injury and proteinuria in transgenic zebrafish
- PMID: 22440901
- PMCID: PMC3358760
- DOI: 10.1681/ASN.2011080776
Inducible podocyte injury and proteinuria in transgenic zebrafish
Abstract
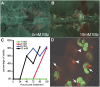
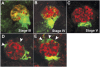
Damage or loss of podocytes causes glomerulosclerosis in murine models, and mutations in podocyte-specific genes cause nephrotic syndrome in humans. Zebrafish provide a valuable model for kidney research, but disruption of pronephroi leads to death within a few days, thereby preventing the study of CKD. In this study, we generated an inducible model of podocyte injury in zebrafish (pod::NTR-mCherry) by expressing a bacterial nitroreductase, which converts metronidazole to a cytotoxin, specifically in podocytes under the control of the zebrafish nphs2/podocin promoter. Application of the prodrug metronidazole to the transgenic fish induces acute damage to the podocytes in pronephroi of larval zebrafish and the mesonephroi of adult zebrafish, resulting in foot-process effacement and podocyte loss. We also developed a functional assay of the glomerular filtration barrier by creating transgenic zebrafish expressing green fluorescent protein (GFP)-tagged vitamin D-binding protein (VDBP) as a tracer for proteinuria. In the VDBP-GFP and pod::NTR-mCherry double-transgenic fish, induction of podocyte damage led to whole-body edema, and the proximal tubules reabsorbed and accumulated VDBP-GFP that leaked through the glomeruli, mimicking the phenotype of human nephrotic syndrome. Moreover, expression of wt1b::GFP, a marker for the developing nephron, extended into the Bowman capsule in response to podocyte injury, suggesting that zebrafish have a podocyte-specific repair process known to occur in mammalian metanephros. These data support the use of these transgenic zebrafish as a model system for studies of glomerular pathogenesis and podocyte regeneration.
Figures




Comment in
-
A photo shoot of proteinuria: zebrafish models of inducible podocyte damage.J Am Soc Nephrol. 2012 Jun;23(6):969-71. doi: 10.1681/ASN.2012040395. Epub 2012 May 10. J Am Soc Nephrol. 2012. PMID: 22581995 No abstract available.
References
-
- Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 - PubMed
-
- Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C: NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000 - PubMed
-
- Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, Ozaltin F, Nürnberg G, Becker C, Hangan D, Pohl M, Kuwertz-Bröking E, Griebel M, Schumacher V, Royer-Pokora B, Bakkaloglu A, Nürnberg P, Zenker M, Hildebrandt F: Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int 70: 1008–1012, 2006 - PubMed
-
- Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A: Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625–2632, 2004 - PubMed
-
- Löwik MM, Groenen PJ, Pronk I, Lilien MR, Goldschmeding R, Dijkman HB, Levtchenko EN, Monnens LA, van den Heuvel LP: Focal segmental glomerulosclerosis in a patient homozygous for a CD2AP mutation. Kidney Int 72: 1198–1203, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

