The circadian clock modulates renal sodium handling
- PMID: 22440902
- PMCID: PMC3358761
- DOI: 10.1681/ASN.2011080842
The circadian clock modulates renal sodium handling
Abstract
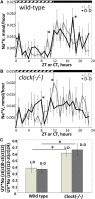
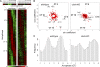
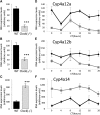
The circadian clock contributes to the control of BP, but the underlying mechanisms remain unclear. We analyzed circadian rhythms in kidneys of wild-type mice and mice lacking the circadian transcriptional activator clock gene. Mice deficient in clock exhibited dramatic changes in the circadian rhythm of renal sodium excretion. In parallel, these mice lost the normal circadian rhythm of plasma aldosterone levels. Analysis of renal circadian transcriptomes demonstrated changes in multiple mechanisms involved in maintaining sodium balance. Pathway analysis revealed the strongest effect on the enzymatic system involved in the formation of 20-HETE, a powerful regulator of renal sodium excretion, renal vascular tone, and BP. This correlated with a significant decrease in the renal and urinary content of 20-HETE in clock-deficient mice. In summary, this study demonstrates that the circadian clock modulates renal function and identifies the 20-HETE synthesis pathway as one of its principal renal targets. It also suggests that the circadian clock affects BP, at least in part, by exerting dynamic control over renal sodium handling.
Figures


References
-
- Doi M, Takahashi Y, Komatsu R, Yamazaki F, Yamada H, Haraguchi S, Emoto N, Okuno Y, Tsujimoto G, Kanematsu A, Ogawa O, Todo T, Tsutsui K, van der Horst GT, Okamura H: Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat Med 16: 67–74, 2010 - PubMed
-
- Wang Q, Maillard M, Schibler U, Burnier M, Gachon F: Cardiac hypertrophy, low blood pressure, and low aldosterone levels in mice devoid of the three circadian PAR bZip transcription factors DBP, HLF, and TEF. Am J Physiol Regul Integr Comp Physiol 299: R1013–R1019, 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

