Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes
- PMID: 22440906
- PMCID: PMC3358767
- DOI: 10.1681/ASN.2011121201
Effects of biocompatible versus standard fluid on peritoneal dialysis outcomes
Abstract
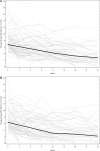
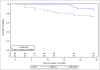
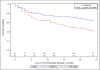
The clinical benefits of using "biocompatible" neutral pH solutions containing low levels of glucose degradation products for peritoneal dialysis compared with standard solutions are uncertain. In this multicenter, open-label, parallel-group, randomized controlled trial, we randomly assigned 185 incident adult peritoneal dialysis patients with residual renal function to use either biocompatible or conventional solution for 2 years. The primary outcome measure was slope of renal function decline. Secondary outcome measures comprised time to anuria, fluid volume status, peritonitis-free survival, technique survival, patient survival, and adverse events. We did not detect a statistically significant difference in the rate of decline of renal function between the two groups as measured by the slopes of GFR: -0.22 and -0.28 ml/min per 1.73 m(2) per month (P=0.17) in the first year in the biocompatible and conventional groups, respectively, and, -0.09 and -0.10 ml/min per 1.73 m(2) per month (P=0.9) in the second year. The biocompatible group exhibited significantly longer times to anuria (P=0.009) and to the first peritonitis episode (P=0.01). This group also had fewer patients develop peritonitis (30% versus 49%) and had lower rates of peritonitis (0.30 versus 0.49 episodes per year, P=0.01). In conclusion, this trial does not support a role for biocompatible fluid in slowing the rate of GFR decline, but it does suggest that biocompatible fluid may delay the onset of anuria and reduce the incidence of peritonitis compared with conventional fluid in peritoneal dialysis.
Figures

Comment in
-
Biocompatible fluid for PD--hanging in the balANZ?Nat Rev Nephrol. 2012 May;8(5):252. doi: 10.1038/nrneph.2012.49. Nat Rev Nephrol. 2012. PMID: 22487702 No abstract available.
References
-
- Krediet RT: 30 years of peritoneal dialysis development: The past and the future. Perit Dial Int 27[Suppl 2]: S35–S41, 2007 - PubMed
-
- Lameire N, Van Biesen W: Epidemiology of peritoneal dialysis: A story of believers and nonbelievers. Nat Rev Nephrol 6: 75–82, 2010 - PubMed
-
- Bargman JM, Thorpe KE, Churchill DN, CANUSA Peritoneal Dialysis Study Group : Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: A reanalysis of the CANUSA study. J Am Soc Nephrol 12: 2158–2162, 2001 - PubMed
-
- Rumpsfeld M, McDonald SP, Johnson DW: Peritoneal small solute clearance is nonlinearly related to patient survival in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 29: 637–646, 2009 - PubMed
-
- Lysaght MJ, Vonesh EF, Gotch F, Ibels L, Keen M, Lindholm B, Nolph KD, Pollock CA, Prowant B, Farrell PC: The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans 37: 598–604, 1991 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

