Memory B cell and other immune responses in children receiving two doses of an oral killed cholera vaccine compared to responses following natural cholera infection in Bangladesh
- PMID: 22441386
- PMCID: PMC3346319
- DOI: 10.1128/CVI.05615-11
Memory B cell and other immune responses in children receiving two doses of an oral killed cholera vaccine compared to responses following natural cholera infection in Bangladesh
Erratum in
- Clin Vaccine Immunol. 2012 Aug;19(8):1337
Abstract
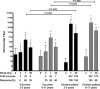
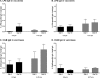
Current oral cholera vaccines induce lower protective efficacy and shorter duration of protection against cholera than wild-type infection provides, and this difference is most pronounced in young children. Despite this, there are limited data comparing immune responses in children following wild-type disease versus vaccination, especially with regard to memory responses associated with long-term immunity. Here, we report a comparison of immune responses in young children (2 to 5 years of age; n = 20) and older children (6 to 17 years of age; n = 20) given two doses of an oral killed cholera vaccine containing recombinant cholera toxin B subunit (CtxB) 14 days apart and compare these responses to those induced in similarly aged children recovering from infection with Vibrio cholerae O1 Ogawa in Bangladesh. We found that the two vaccine groups had comparable vibriocidal and lipopolysaccharide (LPS)-specific plasma antibody responses. Vaccinees developed lower levels of IgG memory B cell (MBC) responses against CtxB but no significant MBC responses against LPS. In contrast, children recovering from natural cholera infection developed prominent LPS IgG and IgA MBC responses, as well as CtxB IgG MBC responses. Plasma LPS IgG, IgA, and IgM responses, as well as vibriocidal responses, were also significantly higher in children following disease than after vaccination. Our findings suggest that acute and memory immune responses following oral cholera vaccination in children are significantly lower than those observed following wild-type disease, especially responses targeting LPS. These findings may explain, in part, the lower efficacy of oral cholera vaccination in children.
Figures

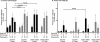


Similar articles
-
Immune responses to the O-specific polysaccharide antigen in children who received a killed oral cholera vaccine compared to responses following natural cholera infection in Bangladesh.Clin Vaccine Immunol. 2013 Jun;20(6):780-8. doi: 10.1128/CVI.00035-13. Epub 2013 Mar 20. Clin Vaccine Immunol. 2013. PMID: 23515016 Free PMC article.
-
Antigen-specific memory B-cell responses in Bangladeshi adults after one- or two-dose oral killed cholera vaccination and comparison with responses in patients with naturally acquired cholera.Clin Vaccine Immunol. 2011 May;18(5):844-50. doi: 10.1128/CVI.00562-10. Epub 2011 Feb 23. Clin Vaccine Immunol. 2011. PMID: 21346055 Free PMC article.
-
Induction of systemic, mucosal and memory antibody responses targeting Vibrio cholerae O1 O-specific polysaccharide (OSP) in adults following oral vaccination with an oral killed whole cell cholera vaccine in Bangladesh.PLoS Negl Trop Dis. 2019 Aug 1;13(8):e0007634. doi: 10.1371/journal.pntd.0007634. eCollection 2019 Aug. PLoS Negl Trop Dis. 2019. PMID: 31369553 Free PMC article.
-
Immune responses to cholera in children.Expert Rev Anti Infect Ther. 2012 Apr;10(4):435-44. doi: 10.1586/eri.12.23. Expert Rev Anti Infect Ther. 2012. PMID: 22512753 Free PMC article. Review.
-
Vibrio cholerae: lessons for mucosal vaccine design.Expert Rev Vaccines. 2011 Jan;10(1):79-94. doi: 10.1586/erv.10.150. Expert Rev Vaccines. 2011. PMID: 21162623 Free PMC article. Review.
Cited by
-
Cholera: Immunity and Prospects in Vaccine Development.J Infect Dis. 2018 Oct 15;218(suppl_3):S141-S146. doi: 10.1093/infdis/jiy414. J Infect Dis. 2018. PMID: 30184117 Free PMC article.
-
Immune responses to the O-specific polysaccharide antigen in children who received a killed oral cholera vaccine compared to responses following natural cholera infection in Bangladesh.Clin Vaccine Immunol. 2013 Jun;20(6):780-8. doi: 10.1128/CVI.00035-13. Epub 2013 Mar 20. Clin Vaccine Immunol. 2013. PMID: 23515016 Free PMC article.
-
From ancient remedies to modern miracles: tracing the evolution of vaccines and their impact on public health.3 Biotech. 2024 Oct;14(10):242. doi: 10.1007/s13205-024-04075-7. Epub 2024 Sep 22. 3 Biotech. 2024. PMID: 39319014 Review.
-
Expanding cholera serosurveillance to vaccinated populations.medRxiv [Preprint]. 2025 Mar 11:2025.03.09.25323598. doi: 10.1101/2025.03.09.25323598. medRxiv. 2025. PMID: 40162250 Free PMC article. Preprint.
-
Antibody Secreting Cell Responses following Vaccination with Bivalent Oral Cholera Vaccine among Haitian Adults.PLoS Negl Trop Dis. 2016 Jun 16;10(6):e0004753. doi: 10.1371/journal.pntd.0004753. eCollection 2016 Jun. PLoS Negl Trop Dis. 2016. PMID: 27308825 Free PMC article.
References
-
- Ahmed T, Svennerholm AM, Al Tarique A, Sultana GN, Qadri F. 2009. Enhanced immunogenicity of an oral inactivated cholera vaccine in infants in Bangladesh obtained by zinc supplementation and by temporary withholding breast-feeding. Vaccine 27:1433–1439 - PubMed
-
- Albert MJ, et al. 2003. Supplementation with zinc, but not vitamin A, improves seroconversion to vibriocidal antibody in children given an oral cholera vaccine. J. Infect. Dis. 187:909–913 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI077883/AI/NIAID NIH HHS/United States
- TW05572/TW/FIC NIH HHS/United States
- TW005572/TW/FIC NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- TW07144/TW/FIC NIH HHS/United States
- K01 TW007144/TW/FIC NIH HHS/United States
- R24 TW007988/TW/FIC NIH HHS/United States
- K01 TW007409/TW/FIC NIH HHS/United States
- K01 TW07409/TW/FIC NIH HHS/United States
- K08 AI089721/AI/NIAID NIH HHS/United States
- U01 AI058935/AI/NIAID NIH HHS/United States
- D43 TW005572/TW/FIC NIH HHS/United States
- R03 AI063079/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

