Acetylcholine induces mesenchymal stem cell migration via Ca2+ /PKC/ERK1/2 signal pathway
- PMID: 22441978
- PMCID: PMC4263956
- DOI: 10.1002/jcb.24148
Acetylcholine induces mesenchymal stem cell migration via Ca2+ /PKC/ERK1/2 signal pathway
Abstract
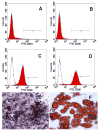
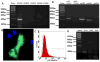
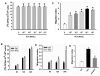
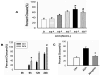
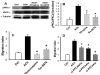
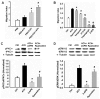
Acetylcholine (ACh) plays an important role in neural and non-neural function, but its role in mesenchymal stem cell (MSC) migration remains to be determined. In the present study, we have found that ACh induces MSC migration via muscarinic acetylcholine receptors (mAChRs). Among several mAChRs, MSCs express mAChR subtype 1 (m1AChR). ACh induces MSC migration via interaction with mAChR1. MEK1/2 inhibitor PD98059 blocks ERK1/2 phosphorylation while partially inhibiting the ACh-induced MSC migration. InsP3Rs inhibitor 2-APB that inhibits MAPK/ERK phosphorylation completely blocks ACh-mediated MSC migration. Interestingly, intracellular Ca(2+) ATPase-specific inhibitor thapsigargin also completely blocks ACh-induced MSC migration through the depletion of intracellular Ca(2+) storage. PKCα or PKCβ inhibitor or their siRNAs only partially inhibit ACh-induced MSC migration, but PKC-ζ siRNA completely inhibits ACh-induced MSC migration via blocking ERK1/2 phosphorylation. These results indicate that ACh induces MSC migration via Ca(2+), PKC, and ERK1/2 signal pathways.
Copyright © 2012 Wiley Periodicals, Inc.
Figures


Similar articles
-
Acetylcholine stimulates cortical precursor cell proliferation in vitro via muscarinic receptor activation and MAP kinase phosphorylation.Eur J Neurosci. 2000 Apr;12(4):1227-40. doi: 10.1046/j.1460-9568.2000.00010.x. Eur J Neurosci. 2000. PMID: 10762352
-
Blocking M2 muscarinic receptor signaling inhibits tumor growth and reverses epithelial-mesenchymal transition (EMT) in non-small cell lung cancer (NSCLC).Cancer Biol Ther. 2015;16(4):634-43. doi: 10.1080/15384047.2015.1029835. Epub 2015 Mar 16. Cancer Biol Ther. 2015. PMID: 25778781 Free PMC article.
-
Muscarinic activation of mitogen-activated protein kinase in rat thyroid epithelial cells.Cell Signal. 2002 Aug;14(8):665-72. doi: 10.1016/s0898-6568(02)00010-4. Cell Signal. 2002. PMID: 12020766
-
Signal transduction in esophageal and LES circular muscle contraction.Yale J Biol Med. 1999 Mar-Jun;72(2-3):153-68. Yale J Biol Med. 1999. PMID: 10780577 Free PMC article. Review.
-
Regulation of Immune Functions by Non-Neuronal Acetylcholine (ACh) via Muscarinic and Nicotinic ACh Receptors.Int J Mol Sci. 2021 Jun 24;22(13):6818. doi: 10.3390/ijms22136818. Int J Mol Sci. 2021. PMID: 34202925 Free PMC article. Review.
Cited by
-
Tetrandrine identified in a small molecule screen to activate mesenchymal stem cells for enhanced immunomodulation.Sci Rep. 2016 Jul 26;6:30263. doi: 10.1038/srep30263. Sci Rep. 2016. PMID: 27457881 Free PMC article.
-
A Novel Endogenous Damage Signal, CSF-2, Activates Multiple Beneficial Functions of Adipose Tissue-Derived Mesenchymal Stem Cells.Mol Ther. 2019 Jun 5;27(6):1087-1100. doi: 10.1016/j.ymthe.2019.03.010. Epub 2019 Mar 19. Mol Ther. 2019. PMID: 30962162 Free PMC article.
-
Airway and lung remodelling in chronic pulmonary obstructive disease: a role for muscarinic receptor antagonists?Drugs. 2015 Jan;75(1):1-8. doi: 10.1007/s40265-014-0319-0. Drugs. 2015. PMID: 25414120 Review.
-
Cinnamtannin B-1 Promotes Migration of Mesenchymal Stem Cells and Accelerates Wound Healing in Mice.PLoS One. 2015 Dec 11;10(12):e0144166. doi: 10.1371/journal.pone.0144166. eCollection 2015. PLoS One. 2015. PMID: 26657737 Free PMC article.
-
Double-edged sword of gonadotropin-releasing hormone (GnRH): A novel role of GnRH in the multiple beneficial functions of endometrial stem cells.Cell Death Dis. 2018 Aug 1;9(8):828. doi: 10.1038/s41419-018-0892-3. Cell Death Dis. 2018. PMID: 30069003 Free PMC article.
References
-
- Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002;14:649–654. - PubMed
-
- Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215–30. - PubMed
-
- Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93:1210–1230. - PubMed
-
- Boss A, Oppitz M, Lippert G, Drews U. Muscarinic cholinergic receptors in the human melanoma cell line SK-Mel 28: modulation of chemotaxis. Clin Exp Dermatol. 2005;30:557–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

