Type-specific interaction between human papillomavirus type 58 E2 protein and E7 protein inhibits E7-mediated oncogenicity
- PMID: 22442110
- PMCID: PMC3542735
- DOI: 10.1099/vir.0.039354-0
Type-specific interaction between human papillomavirus type 58 E2 protein and E7 protein inhibits E7-mediated oncogenicity
Abstract
Human papillomavirus type 58 (HPV-58) is a very common HPV type in eastern Asia. Little is known about its biology and tumorigenesis. In this study, HPV-58 E2 protein (58E2) was found to interact with E7 protein (58E7), and the hinge domain of 58E2 was shown to be responsible for binding to the 58E7 protein. Interestingly, the E2-E7 interaction appears to be HPV type-specific, as we found that the HPV-16 E2 could not bind to the 58E7 protein, and neither did 58E2 interact with HPV-16 E7. The biological consequence(s) of the E2-E7 interaction in HPV-58, especially in viral tumorigenesis, was investigated. Results showed that, through interacting with 58E7, 58E2 prevented E7-induced retinoblastoma protein (pRb) degradation and prolonged the half-life of pRb in cells. Additionally, 58E2 abrogated 58E7-induced cell proliferation. These observations collectively suggest that direct interaction with 58E7 is another mechanism for 58E2 to inhibit 58E7-associated carcinogenesis in addition to regulating expression of the 58E7 gene.
Figures
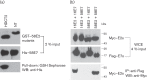

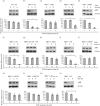


Similar articles
-
Regulation of human papillomavirus type 16 E7 activity through direct protein interaction with the E2 transcriptional activator.J Virol. 2006 Feb;80(4):1787-97. doi: 10.1128/JVI.80.4.1787-1797.2006. J Virol. 2006. PMID: 16439535 Free PMC article.
-
Comparison of the binding of the human papillomavirus type 16 and cottontail rabbit papillomavirus E7 proteins to the retinoblastoma gene product.J Gen Virol. 1993 Jan;74 ( Pt 1):115-9. doi: 10.1099/0022-1317-74-1-115. J Gen Virol. 1993. PMID: 8380832
-
Human papillomavirus type 45 E7 is a transforming protein inducing retinoblastoma protein degradation and anchorage-independent cell cycle progression.Virology. 2008 Sep 15;379(1):20-9. doi: 10.1016/j.virol.2008.06.004. Epub 2008 Jul 22. Virology. 2008. PMID: 18649911
-
Interactions of HPV E6 and E7 oncoproteins with tumour suppressor gene products.Cancer Surv. 1992;12:197-217. Cancer Surv. 1992. PMID: 1322242 Review.
-
Molecular mechanisms of transformation by the human papillomaviruses.Princess Takamatsu Symp. 1989;20:199-206. Princess Takamatsu Symp. 1989. PMID: 2562182 Review.
Cited by
-
The papillomavirus E7 proteins.Virology. 2013 Oct;445(1-2):138-68. doi: 10.1016/j.virol.2013.04.013. Epub 2013 May 31. Virology. 2013. PMID: 23731972 Free PMC article. Review.
-
Human Papillomavirus (HPV)-associated Oral Cancers and Treatment Strategies.J Dent Res. 2014 Jul;93(7 Suppl):29S-36S. doi: 10.1177/0022034514527969. Epub 2014 Mar 24. J Dent Res. 2014. PMID: 24663683 Free PMC article. Review.
References
-
- Boyer S. N., Wazer D. E., Band V. (1996). E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res 56, 4620–4624 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

