Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation
- PMID: 22442146
- PMCID: PMC3340216
- DOI: 10.1074/jbc.C112.353946
Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation
Abstract
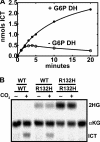
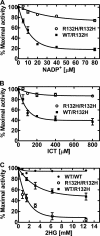
Isocitrate dehydrogenase (IDH) is a reversible enzyme that catalyzes the NADP(+)-dependent oxidative decarboxylation of isocitrate (ICT) to α-ketoglutarate (αKG) and the NADPH/CO(2)-dependent reductive carboxylation of αKG to ICT. Reductive carboxylation by IDH1 was potently inhibited by NADP(+) and, to a lesser extent, by ICT. IDH1 and IDH2 with cancer-associated mutations at the active site arginines were unable to carry out the reductive carboxylation of αKG. These mutants were also defective in ICT decarboxylation and converted αKG to 2-hydroxyglutarate using NADPH. These mutant proteins were thus defective in both of the normal reactions of IDH. Biochemical analysis of heterodimers between wild-type and mutant IDH1 subunits showed that the mutant subunit did not inactivate reductive carboxylation by the wild-type subunit. Cells expressing the mutant IDH are thus deficient in their capacity for reductive carboxylation and may be compromised in their ability to produce acetyl-CoA under hypoxia or when mitochondrial function is otherwise impaired.
Figures


References
-
- Ochoa S. (1948) Biosynthesis of tricarboxylic acids by carbon dioxide fixation. III. Enzymatic mechanisms. J. Biol. Chem. 174, 133–157 - PubMed
-
- D'Adamo A. F., Jr., Haft D. E. (1965) An alternate pathway of α-ketoglutarate catabolism in the isolated, perfused rat liver. I. Studies with dl-glutamate-2- and -5-14C. J. Biol. Chem. 240, 613–617 - PubMed
-
- Ramachandran N., Colman R. F. (1980) Chemical characterization of distinct subunits of pig heart DPN-specific isocitrate dehydrogenase. J. Biol. Chem. 255, 8859–8864 - PubMed
-
- Northrop D. B., Cleland W. W. (1974) The kinetics of pig heart triphosphopyridine nucleotide-isocitrate dehydrogenase. II. Dead-end and multiple inhibition studies. J. Biol. Chem. 249, 2928–2931 - PubMed
-
- Uhr M. L., Thompson V. W., Cleland W. W. (1974) The kinetics of pig heart triphosphopyridine nucleotide-isocitrate dehydrogenase. I. Initial velocity, substrate and product inhibition, and isotope exchange studies. J. Biol. Chem. 249, 2920–2927 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

