Cell type-specific β2-adrenergic receptor clusters identified using photoactivated localization microscopy are not lipid raft related, but depend on actin cytoskeleton integrity
- PMID: 22442147
- PMCID: PMC3351334
- DOI: 10.1074/jbc.M111.329912
Cell type-specific β2-adrenergic receptor clusters identified using photoactivated localization microscopy are not lipid raft related, but depend on actin cytoskeleton integrity
Abstract
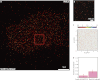
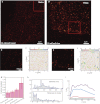
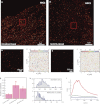
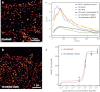
Recent developments in the field of optical super-resolution techniques allow both a 10-fold increase in resolution as well as an increased ability to quantify the number of labeled molecules visualized in the fluorescence measurement. By using photoactivated localization microscopy (PALM) and an experimental approach based on the systematic comparison with a nonclustering peptide as a negative control, we found that the prototypical G protein-coupled receptor β2-adrenergic receptor is partially preassociated in nanoscale-sized clusters only in the cardiomyocytes, such as H9C2 cells, but not in other cell lines, such as HeLa and Chinese hamster ovary (CHO). The addition of the agonist for very short times or the addition of the inverse agonist did not significantly affect the organization of receptor assembly. To investigate the mechanism governing cluster formation, we altered plasma membrane properties with cholesterol removal and actin microfilament disruption. Although cholesterol is an essential component of cell membranes and it is supposed to be enriched in the lipid rafts, its sequestration and removal did not affect receptor clustering, whereas the inhibition of actin polymerization did decrease the number of clusters. Our findings are therefore consistent with a model in which β2 receptor clustering is influenced by the actin cytoskeleton, but it does not rely on lipid raft integrity, thus ruling out the possibility that cell type-specific β2 receptor clustering is associated with the raft.
Figures

References
-
- Patel H. H., Murray F., Insel P. A. (2008) G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb. Exp. Pharmacol. 186, 167–184 - PubMed
-
- Jacobson K., Mouritsen O. G., Anderson R. G. (2007) Lipid rafts, at a crossroad between cell biology and physics. Nat. Cell Biol. 9, 7–14 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

