Interleukin enhancer-binding factor 3/NF110 is a target of YM155, a suppressant of survivin
- PMID: 22442257
- PMCID: PMC3394938
- DOI: 10.1074/mcp.M111.013243
Interleukin enhancer-binding factor 3/NF110 is a target of YM155, a suppressant of survivin
Abstract
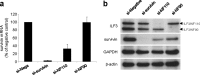
Survivin is responsible for cancer progression and drug resistance in many types of cancer. YM155 selectively suppresses the expression of survivin and induces apoptosis in cancer cells in vitro and in vivo. However, the mechanism underlying these effects of YM155 is unknown. Here, we show that a transcription factor, interleukin enhancer-binding factor 3 (ILF3)/NF110, is a direct binding target of YM155. The enhanced survivin promoter activity by overexpression of ILF3/NF110 was attenuated by YM155 in a concentration-dependent manner, suggesting that ILF3/NF110 is the physiological target through which YM155 mediates survivin suppression. The results also show that the unique C-terminal region of ILF3/NF110 is important for promoting survivin expression and for high affinity binding to YM155.
Figures




References
-
- Tamm I., Wang Y., Sausville E., Scudiero D. A., Vigna N., Oltersdorf T., Reed J. C. (1998) IAP-family protein Survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 58, 5315–5320 - PubMed
-
- Altieri D. C. (2006) The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr. Opin. Cell Biol. 18, 609–615 - PubMed
-
- Lens S. M., Vader G., Medema R. H. (2006) The case for Survivin as mitotic regulator. Curr. Opin. Cell Biol. 18, 616–622 - PubMed
-
- Ambrosini G., Adida C., Altieri D. C. (1997) A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 3, 917–921 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

