Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study
- PMID: 22442396
- PMCID: PMC3308305
- DOI: 10.2337/dc11-1299
Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study
Abstract
Objective: Metformin produced weight loss and delayed or prevented diabetes in the Diabetes Prevention Program (DPP). We examined its long-term safety and tolerability along with weight loss, and change in waist circumference during the DPP and its long-term follow-up.
Research design and methods: The randomized double-blind clinical trial of metformin or placebo followed by a 7-8-year open-label extension and analysis of adverse events, tolerability, and the effect of adherence on change in weight and waist circumference.
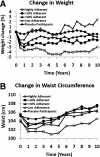
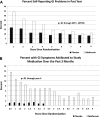
Results: No significant safety issues were identified. Gastrointestinal symptoms were more common in metformin than placebo participants and declined over time. During the DPP, average hemoglobin and hematocrit levels were slightly lower in the metformin group than in the placebo group. Decreases in hemoglobin and hematocrit in the metformin group occurred during the first year following randomization, with no further changes observed over time. During the DPP, metformin participants had reduced body weight and waist circumference compared with placebo (weight by 2.06 ± 5.65% vs. 0.02 ± 5.52%, P < 0.001, and waist circumference by 2.13 ± 7.06 cm vs. 0.79 ± 6.54 cm, P < 0.001 in metformin vs. placebo, respectively). The magnitude of weight loss during the 2-year double-blind period was directly related to adherence (P < 0.001). Throughout the unblinded follow-up, weight loss remained significantly greater in the metformin group than in the placebo group (2.0 vs. 0.2%, P < 0.001), and this was related to the degree of continuing metformin adherence (P < 0.001).
Conclusions: Metformin used for diabetes prevention is safe and well tolerated. Weight loss is related to adherence to metformin and is durable for at least 10 years of treatment.
Trial registration: ClinicalTrials.gov NCT00004992 NCT00038727.
Figures

Comment in
-
Steps toward the meaningful translation of prevention strategies for type 2 diabetes.Diabetes Care. 2012 Apr;35(4):663-5. doi: 10.2337/dc12-0119. Diabetes Care. 2012. PMID: 22442393 Free PMC article. No abstract available.
References
-
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 1999;131:281–303 - PubMed
-
- UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 - PubMed
-
- Bray GA, Greenway FL. Pharmacological treatment of the overweight patient. Pharmacol Rev 2007;59:151–184 - PubMed

