Antigen recognition is facilitated by invadosome-like protrusions formed by memory/effector T cells
- PMID: 22442443
- PMCID: PMC3324627
- DOI: 10.4049/jimmunol.1102594
Antigen recognition is facilitated by invadosome-like protrusions formed by memory/effector T cells
Abstract
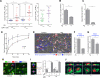
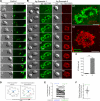
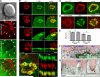
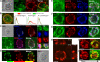
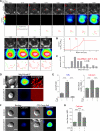
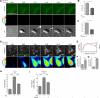
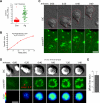
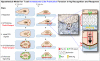
Adaptive immunity requires that T cells efficiently scan diverse cell surfaces to identify cognate Ag. However, the basic cellular mechanisms remain unclear. In this study, we investigated this process using vascular endothelial cells, APCs that possess a unique and extremely advantageous, planar morphology. High-resolution imaging revealed that CD4 memory/effector T cells dynamically probe the endothelium by extending submicron-scale, actin-rich "invadosome/podosome-like protrusions" (ILPs). The intimate intercellular contacts enforced by ILPs consistently preceded and supported T cell activation in response to endothelial MHC class II/Ag. The resulting calcium flux stabilized dense arrays of ILPs (each enriched in TCR, protein kinase C-θ, ZAP70, phosphotyrosine, and HS1), forming what we term a podo-synapse. Similar findings were made using CD8 CTLs on endothelium. Furthermore, careful re-examination of both traditional APC models and professional APCs suggests broad relevance for ILPs in facilitating Ag recognition. Together, our results indicate that ILPs function as sensory organelles that serve as actuators of immune surveillance.
Figures

References
-
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. - PubMed
-
- Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annual review of immunology. 2006;24:419–466. - PubMed
-
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annual review of biomedical engineering. 2007;9:121–167. - PubMed
-
- Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

