Theoretical principles underlying optical stimulation of a channelrhodopsin-2 positive pyramidal neuron
- PMID: 22442566
- PMCID: PMC3378402
- DOI: 10.1152/jn.00501.2011
Theoretical principles underlying optical stimulation of a channelrhodopsin-2 positive pyramidal neuron
Abstract
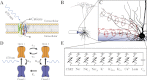
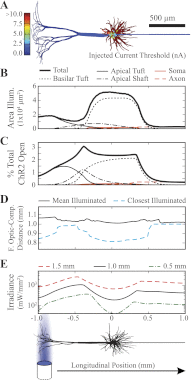
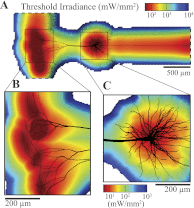
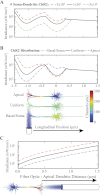
Optogenetics is an emerging field of neuromodulation that permits scaled, millisecond temporal control of the membrane dynamics of genetically targeted cells using light. Optogenetic technology has revolutionized neuroscience research; however, numerous biophysical questions remain on the optical and neuronal factors impacting the modulation of neural activity with photon-sensitive ion channels. To begin to address such questions, we developed a computational tool to explore the underlying principles of optogenetic neural stimulation. This "light-neuron" model consists of theoretical representations of the light dynamics generated by a fiber optic in brain tissue, coupled to a multicompartment cable model of a cortical pyramidal neuron embedded with channelrhodopsin-2 (ChR2) membrane dynamics. Simulations revealed that the large energies required to generate an action potential are primarily due to the limited conductivity of ChR2, and that the major determinants of stimulation threshold are the surface area of illuminated cell membrane and proximity to the light source. Our results predict that the activation threshold is sensitive to many of the properties of ChR2 (density, conductivity, and kinetics), tissue medium (scattering and absorbance), and the fiber-optic light source (diameter and numerical aperture). We also illustrate the impact of redistributing the ChR2 expression density (uniform vs. nonuniform) on the activation threshold. The model system developed in this study represents a scientific instrument to characterize the effects of optogenetic neuromodulation, as well as an engineering design tool to help guide future development of optogenetic technology.
Figures






References
-
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4: S143–S156, 2007 - PubMed
-
- Carnevale NT, Hines ML. The NEURON Book (1st Ed.). Cambridge, UK: Cambridge University Press, 2009
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

