Regulation of presynaptic strength by controlling Ca2+ channel mobility: effects of cholesterol depletion on release at the cone ribbon synapse
- PMID: 22442573
- PMCID: PMC3378415
- DOI: 10.1152/jn.00779.2011
Regulation of presynaptic strength by controlling Ca2+ channel mobility: effects of cholesterol depletion on release at the cone ribbon synapse
Abstract
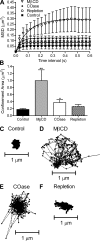
Synaptic communication requires proper coupling between voltage-gated Ca(2+) (Ca(V)) channels and synaptic vesicles. In photoreceptors, L-type Ca(V) channels are clustered close to synaptic ribbon release sites. Although clustered, Ca(V) channels move continuously within a confined domain slightly larger than the base of the ribbon. We hypothesized that expanding Ca(V) channel confinement domains should increase the number of channel openings needed to trigger vesicle release. Using single-particle tracking techniques, we measured the expansion of Ca(V) channel confinement domains caused by depletion of membrane cholesterol with cholesterol oxidase or methyl-β-cyclodextrin. With paired whole cell recordings from cones and horizontal cells, we then determined the number of Ca(V) channel openings contributing to cone Ca(V) currents (I(Ca)) and the number of vesicle fusion events contributing to horizontal cell excitatory postsynaptic currents (EPSCs) following cholesterol depletion. Expansion of Ca(V) channel confinement domains reduced the peak efficiency of release, decreasing the number of vesicle fusion events accompanying opening of each Ca(V) channel. Cholesterol depletion also inhibited exocytotic capacitance increases evoked by brief depolarizing steps. Changes in efficiency were not due to changes in I(Ca) amplitude or glutamate receptor properties. Replenishing cholesterol restored Ca(V) channel domain size and release efficiency to control levels. These results indicate that cholesterol is important for organizing the cone active zone. Furthermore, the finding that cholesterol depletion impairs coupling between channel opening and vesicle release by allowing Ca(V) channels to move further from release sites shows that changes in presynaptic Ca(V) channel mobility can be a mechanism for adjusting synaptic strength.
Figures



References
-
- Abulrob A, Tauskela JS, Mealing G, Brunette E, Faid K, Stanimirovic D. Protection by cholesterol-extracting cyclodextrins, a role for N-methyl-d-aspartate receptor redistribution. J Neurochem 92: 1477–1486, 2005 - PubMed
-
- Aguirre GD, Acland GM, Maude MB, Anderson RE. Diets enriched in docosahexaenoic acid fail to correct progressive rod-cone degeneration (prcd) phenotype. Invest Ophthalmol Vis Sci 38: 2387–2407, 1997 - PubMed
-
- Ait-Haddou R, Kurachi Y, Nomura T. On calcium-buffer dynamics within the excess buffer regime. J Theor Biol 264: 55–65, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

