Mechanism of RPE cell death in α-crystallin deficient mice: a novel and critical role for MRP1-mediated GSH efflux
- PMID: 22442691
- PMCID: PMC3307734
- DOI: 10.1371/journal.pone.0033420
Mechanism of RPE cell death in α-crystallin deficient mice: a novel and critical role for MRP1-mediated GSH efflux
Abstract
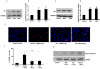
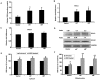
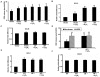
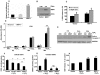
Absence of α-crystallins (αA and αB) in retinal pigment epithelial (RPE) cells renders them susceptible to oxidant-induced cell death. We tested the hypothesis that the protective effect of α-crystallin is mediated by changes in cellular glutathione (GSH) and elucidated the mechanism of GSH efflux. In α-crystallin overexpressing cells resistant to cell death, cellular GSH was >2 fold higher than vector control cells and this increase was seen particularly in mitochondria. The high GSH levels associated with α-crystallin overexpression were due to increased GSH biosynthesis. On the other hand, cellular GSH was decreased by 50% in murine retina lacking αA or αB crystallin. Multiple multidrug resistance protein (MRP) family isoforms were expressed in RPE, among which MRP1 was the most abundant. MRP1 was localized to the plasma membrane and inhibition of MRP1 markedly decreased GSH efflux. MRP1-suppressed cells were resistant to cell death and contained elevated intracellular GSH and GSSG. Increased GSH in MRP1-supressed cells resulted from a higher conversion of GSSG to GSH by glutathione reductase. In contrast, GSH efflux was significantly higher in MRP1 overexpressing RPE cells which also contained lower levels of cellular GSH and GSSG. Oxidative stress further increased GSH efflux with a decrease in cellular GSH and rendered cells apoptosis-prone. In conclusion, our data reveal for the first time that 1) MRP1 mediates GSH and GSSG efflux in RPE cells; 2) MRP1 inhibition renders RPE cells resistant to oxidative stress-induced cell death while MRP1 overexpression makes them susceptible and 3) the antiapoptotic function of α-crystallin in oxidatively stressed cells is mediated in part by GSH and MRP1. Our findings suggest that MRP1 and α crystallin are potential therapeutic targets in pathological retinal degenerative disorders linked to oxidative stress.
Conflict of interest statement
Figures




References
-
- Cai X, McGinnis JF. Oxidative stress: the achilles' heel of neurodegenerative diseases of the retina. Front Biosci. 2012;17:1976–1995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

