Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects
- PMID: 22443121
- PMCID: PMC3397118
- DOI: 10.1089/ten.TEA.2011.0325
Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects
Abstract
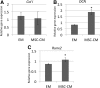
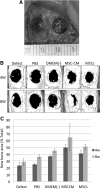
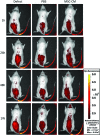
Tissue engineering has recently become available as a treatment procedure for bone augmentation. However, this procedure has several problems, such as high capital investment and expensive cell culture, complicated safety and quality management issues regarding cell handling, and patient problems with the invasive procedure of cell collection. Moreover, it was reported that stem cells secrete many growth factors and chemokines during their cultivation, which could affect cellular characteristics and behavior. This study investigated the effect of stem-cell-cultured conditioned media on bone regeneration. Cultured conditioned media from human bone marrow-derived mesenchymal stem cells (MSC-CM) enhanced the migration, proliferation, and expression of osteogenic marker genes, such as osteocalcin and Runx2, of rat MSCs (rMSCs) in vitro. MSC-CM includes cytokines such as insulin-like growth factor-1 and vascular endothelial growth factor. In vivo, a prepared bone defect of a rat calvarial model was implanted in five different rat groups using one of the following graft materials: human MSCs/agarose (MSCs), MSC-CM/agarose (MSC-CM), Dulbecco's modified Eagle's medium without serum [DMEM(-)]/agarose [DMEM(-)], PBS/agarose (PBS), and defect only (Defect). After 4 and 8 weeks, implant sections were evaluated using microcomputed tomography (micro-CT) and histological analysis. Micro-CT analysis indicated that the MSC-CM group had a greater area of newly regenerated bone compared with the other groups (p<0.05) and histological analysis at 8 weeks indicated that the newly regenerated bone bridge almost covered the defect. Interestingly, the effects of MSC-CM were stronger than those of the MSC group. In vivo imaging and immunohistochemical staining of transgenic rats expressing green fluorescent protein also showed that migration of rMSCs to the bone defect in the MSC-CM group was greater than in the other groups. These results demonstrated that MSC-CM can regenerate bone through mobilization of endogenous stem cells. The use of stem-cell-cultured conditioned media for bone regeneration is a unique concept that utilizes paracrine factors of stem cells without cell transplantation.
Figures



References
-
- Barome A. Covani U. Maxilary alveolar ridge reconstruction with nonvasculized autogenous block bone: clinical results. J Oral Maxillofac Surg. 2007;65:2039. - PubMed
-
- Kurz L.T. Grafin S.R. Booth R.E. Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324. - PubMed
-
- Athanasiou V.T. Papachristou D.J. Panagopoulos A. Saridis A. Scopa C.D. Megas P. Histological comparison of autograft, allograft-DBM, xenograft, and synthetic grafts in a trabecular bone defect: an experimental study in rabbits. Med Sci Monit. 2010;16:BR24. - PubMed
-
- Eppley B.L. Pietzak W.S. Blanton M.W. Allograft and alloplastic bone substitutes: a review of science and thchnology for the craniomaxillofacial surgeon. J Craniofac Surg. 2005;16:981. - PubMed
-
- Moore W. Graves S.E. Bain G.I. Synthetic bone graft substitutes. ANZ J Surg. 2001;71:354. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

