Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity
- PMID: 22443164
- PMCID: PMC3371128
- DOI: 10.1111/j.1471-4159.2012.07739.x
Genetic depletion of brain 5HT reveals a common molecular pathway mediating compulsivity and impulsivity
Abstract
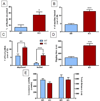
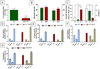
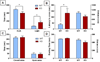
Neuropsychiatric disorders characterized by behavioral disinhibition, including disorders of compulsivity (e.g. obsessive-compulsive disorder; OCD) and impulse-control (e.g. impulsive aggression), are severe, highly prevalent and chronically disabling. Treatment options for these diseases are extremely limited. The pathophysiological bases of disorders of behavioral disinhibition are poorly understood but it has been suggested that serotonin dysfunction may play a role. Mice lacking the gene encoding brain tryptophan hydroxylase 2 (Tph2-/-), the initial and rate-limiting enzyme in the synthesis of serotonin, were tested in numerous behavioral assays that are well known for their utility in modeling human neuropsychiatric diseases. Mice lacking Tph2 (and brain 5HT) show intense compulsive and impulsive behaviors to include extreme aggression. The impulsivity is motor in form and not cognitive because Tph2-/- mice show normal acquisition and reversal learning on a spatial learning task. Restoration of 5HT levels by treatment of Tph2-/- mice with its immediate precursor 5-hydroxytryptophan attenuated compulsive and impulsive-aggressive behaviors. Surprisingly, in Tph2-/- mice, the lack of 5HT was not associated with anxiety-like behaviors. The results indicate that 5HT mediates behavioral disinhibition in the mammalian brain independent of anxiogenesis.
© 2012 The Authors. Journal of Neurochemistry © 2012 International Society for Neurochemistry.
Conflict of interest statement
The authors declare that they have no conflict of interest related to the publication of this article.
Figures


References
-
- Albelda N, Joel D. Animal models of obsessive-compulsive disorder: Exploring pharmacology and neural substrates. Neurosci. Biobehav. Rev. 2012;36:47–63. - PubMed
-
- Bevilacqua L, Doly S, Kaprio J, Yuan Q, Tikkanen R, Paunio T, Zhou Z, Wedenoja J, Maroteaux L, Diaz S, Belmer A, Hodgkinson CA, Dell'osso L, Suvisaari J, Coccaro E, Rose RJ, Peltonen L, Virkkunen M, Goldman D. A population-specific HTR2B stop codon predisposes to severe impulsivity. Nature. 2010;468:1061–1066. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

