OsWRKY22, a monocot WRKY gene, plays a role in the resistance response to blast
- PMID: 22443363
- PMCID: PMC6638809
- DOI: 10.1111/j.1364-3703.2012.00795.x
OsWRKY22, a monocot WRKY gene, plays a role in the resistance response to blast
Abstract
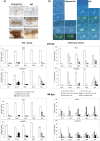
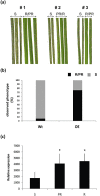
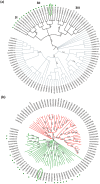
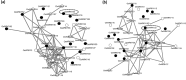
With the aim of identifying novel regulators of host and nonhost resistance to fungi in rice, we carried out a systematic mutant screen of mutagenized lines. Two mutant wrky22 knockout lines revealed clear-cut enhanced susceptibility to both virulent and avirulent Magnaporthe oryzae strains and altered cellular responses to nonhost Magnaporthe grisea and Blumeria graminis fungi. In addition, the analysis of the pathogen responses of 24 overexpressor OsWRKY22 lines revealed enhanced resistance phenotypes on infection with virulent M. oryzae strain, confirming that OsWRKY22 is involved in rice resistance to blast. Bioinformatic analyses determined that the OsWRKY22 gene belongs to a well-defined cluster of monocot-specific WRKYs. The co-regulatory analysis revealed no significant co-regulation of OsWRKY22 with a representative panel of OsWRKYs, supporting its unique role in a series of transcriptional responses. In contrast, inquiring a subset of biotic stress-related Affymetrix data, a large number of resistance and defence-related genes were found to be putatively co-expressed with OsWRKY22. Taken together, all gathered experimental evidence places the monocot-specific OsWRKY22 gene at the convergence point of signal transduction circuits in response to both host and nonhost fungi encountering rice plants.
© 2012 PARCO TECHNOLOGICO PADANO. MOLECULAR PLANT PATHOLOGY © 2012 BSPP AND BLACKWELL PUBLISHING LTD.
Figures



References
-
- Abuqamar, S. , Chen, X. , Dhawan, R. , Bluhm, B. , Salmeron, J. , Lam, S. , Dietrich, R.A. and Mengiste, T. (2006) Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J. 48, 28–44. - PubMed
-
- Bolstad, B. , Irizarry, R. , Strand, M. and Speed, T. (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics, 19, 185–193. - PubMed
-
- Chujo, T. , Takai, R. , Akimoto‐Tomiyama, C. , Ando, S. , Minami, E. , Nagamura, Y. , Kaku, H. , Shibuya, N. , Yasuda, M. , Nakashita, H. , Umemura, K. , Okada, A. , Okada, K. , Nojiri, H. and Yamane, H. (2007) Involvement of the elicitor‐induced gene OsWRKY53 in the expression of defense related genes in rice. Biochim. Biophys. Acta, 1769, 497–505. - PubMed
-
- Collins, N.C. , Thordal‐Christensen, H. , Lipka, V. , Bau, S. , Kombrink, E. , Qiu, J.L. , Hückelhoven, R. , Stein, M. , Freialdenhoven, A. , Somerville, S.C. and Schulze‐Lefert, P. (2003) SNARE‐protein‐mediated disease resistance at the plant cell wall. Nature, 425, 973–977. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

