Mechanisms of gamma oscillations
- PMID: 22443509
- PMCID: PMC4049541
- DOI: 10.1146/annurev-neuro-062111-150444
Mechanisms of gamma oscillations
Abstract
Gamma rhythms are commonly observed in many brain regions during both waking and sleep states, yet their functions and mechanisms remain a matter of debate. Here we review the cellular and synaptic mechanisms underlying gamma oscillations and outline empirical questions and controversial conceptual issues. Our main points are as follows: First, gamma-band rhythmogenesis is inextricably tied to perisomatic inhibition. Second, gamma oscillations are short-lived and typically emerge from the coordinated interaction of excitation and inhibition, which can be detected as local field potentials. Third, gamma rhythm typically concurs with irregular firing of single neurons, and the network frequency of gamma oscillations varies extensively depending on the underlying mechanism. To document gamma oscillations, efforts should be made to distinguish them from mere increases of gamma-band power and/or increased spiking activity. Fourth, the magnitude of gamma oscillation is modulated by slower rhythms. Such cross-frequency coupling may serve to couple active patches of cortical circuits. Because of their ubiquitous nature and strong correlation with the "operational modes" of local circuits, gamma oscillations continue to provide important clues about neuronal population dynamics in health and disease.
Figures
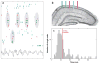


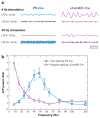
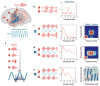
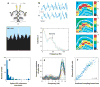

References
LITERATURE CITED
-
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. - PubMed
RELATED RESOURCES
-
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. - PubMed
-
- Engel A, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–16. - PubMed
-
- György Buzsáki’s Web page. http://www.med.nyu.edu/buzsakilab/
-
- Uhlhaas P, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–68. - PubMed
-
- Varela F, Lachaux J, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

