Tryptophan metabolism in breast cancers: molecular imaging and immunohistochemistry studies
- PMID: 22444239
- PMCID: PMC3443322
- DOI: 10.1016/j.nucmedbio.2012.01.010
Tryptophan metabolism in breast cancers: molecular imaging and immunohistochemistry studies
Abstract
Introduction: Tryptophan oxidation via the kynurenine pathway is an important mechanism of tumoral immunoresistance. Increased tryptophan metabolism via the serotonin pathway has been linked to malignant progression in breast cancer. In this study, we combined quantitative positron emission tomography (PET) with tumor immunohistochemistry to analyze tryptophan transport and metabolism in breast cancer.
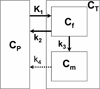
Methods: Dynamic α-[(11)C]methyl-l-tryptophan (AMT) PET was performed in nine women with stage II-IV breast cancer. PET tracer kinetic modeling was performed in all tumors. Expression of L-type amino acid transporter 1 (LAT1), indoleamine 2,3-dioxygenase (IDO; the initial and rate-limiting enzyme of the kynurenine pathway) and tryptophan hydroxylase 1 (TPH1; the initial enzyme of the serotonin pathway) was assessed by immunostaining of resected tumor specimens.
Results: Tumor AMT uptake peaked at 5-20 min postinjection in seven tumors; the other two cases showed protracted tracer accumulation. Tumor standardized uptake values (SUVs) varied widely (2.6-9.8) and showed a strong positive correlation with volume of distribution values derived from kinetic analysis (P<.01). Invasive ductal carcinomas (n=6) showed particularly high AMT SUVs (range, 4.7-9.8). Moderate to strong immunostaining for LAT1, IDO and TPH1 was detected in most tumor cells.
Conclusions: Breast cancers show differential tryptophan kinetics on dynamic PET. SUVs measured 5-20 min postinjection reflect reasonably the tracer's volume of distribution. Further studies are warranted to determine if in vivo AMT accumulation in these tumors is related to tryptophan metabolism via the kynurenine and serotonin pathways.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





References
-
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. - PubMed
-
- Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. - PubMed
-
- Travers MT, Gow IF, Barber MC, Thomson J, Shennan DB. Indoleamine 2,3-dioxygenase activity and L-tryptophan transport in human breast cancer cells. Biochim Biophys Acta. 2004;1661:106–112. - PubMed
-
- Sakurai K, Amano S, Enomoto K, Kashio M, Saito Y, Sakamoto A, et al. Study of indoleamine 2,3-dioxygenase expression in patients with breast cancer. Gan To Kagaku Ryoho. 2005;32:1546–1549. - PubMed
-
- Jacquemier J, Bertucci F, Finetti P, Esterni B, Charafe-Jauffret E, Thibult ML, et al. High expression of indoleamine 2,3-dioxygenase in the tumour is associated with medullary features and favourable outcome in basal-like breast carcinoma. Int J Cancer. 2012;130:96–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

