Free extracellular diffusion creates the Dpp morphogen gradient of the Drosophila wing disc
- PMID: 22445299
- PMCID: PMC3338872
- DOI: 10.1016/j.cub.2012.02.065
Free extracellular diffusion creates the Dpp morphogen gradient of the Drosophila wing disc
Abstract
Background: How morphogen gradients form has long been a subject of controversy. The strongest support for the view that morphogens do not simply spread by free diffusion has come from a variety of studies of the Decapentaplegic (Dpp) gradient of the Drosophila larval wing disc.
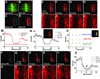
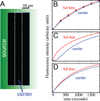
Results: In the present study, we initially show how the failure, in such studies, to consider the coupling of transport to receptor-mediated uptake and degradation has led to estimates of transport rates that are orders of magnitude too low, lending unwarranted support to a variety of hypothetical mechanisms, such as "planar transcytosis" and "restricted extracellular diffusion." Using several independent dynamic methods, we obtain data that are inconsistent with such models and show directly that Dpp transport occurs by simple, rapid diffusion in the extracellular space. We discuss the implications of these findings for other morphogen systems in which complex transport mechanisms have been proposed.
Conclusions: We believe that these findings resolve a major, longstanding question about morphogen gradient formation and provide a solid framework for interpreting experimental observations of morphogen gradient dynamics.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Crick FHC. Diffusion in Embryogenesis. Nature. 1970;225:420–422. - PubMed
-
- Lander AD, Nie Q, Wan FY. Do morphogen gradients arise by diffusion? Dev Cell. 2002;2:785–796. - PubMed
-
- Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature. 2002;419:304–308. - PubMed
-
- Eldar A, Rosin D, Shilo BZ, Barkai N. Self-enhanced ligand degradation underlies robustness of morphogen gradients. Dev Cell. 2003;5:635–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

