Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation
- PMID: 22445340
- PMCID: PMC3551478
- DOI: 10.1016/j.neuron.2012.02.009
Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation
Abstract
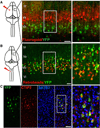
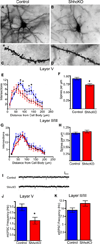
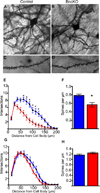
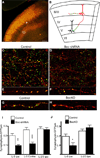
The precise connectivity of inputs and outputs is critical for cerebral cortex function; however, the cellular mechanisms that establish these connections are poorly understood. Here, we show that the secreted molecule Sonic Hedgehog (Shh) is involved in synapse formation of a specific cortical circuit. Shh is expressed in layer V corticofugal projection neurons and the Shh receptor, Brother of CDO (Boc), is expressed in local and callosal projection neurons of layer II/III that synapse onto the subcortical projection neurons. Layer V neurons of mice lacking functional Shh exhibit decreased synapses. Conversely, the loss of functional Boc leads to a reduction in the strength of synaptic connections onto layer Vb, but not layer II/III, pyramidal neurons. These results demonstrate that Shh is expressed in postsynaptic target cells while Boc is expressed in a complementary population of presynaptic input neurons, and they function to guide the formation of cortical microcircuitry.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Sonic hedgehog, BOC, and synaptic development: new players for an old game.Neuron. 2012 Mar 22;73(6):1055-8. doi: 10.1016/j.neuron.2012.03.008. Epub 2012 Mar 21. Neuron. 2012. PMID: 22445332
References
-
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
-
- Alcamo EA, Chirivella L, Dautzenberg M, Dobreva G, Fariñas I, Grosschedl R, McConnell SK. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. - PubMed
-
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. - PubMed
-
- Britanova O, de Juan Romero C, Cheung A, Kwan KY, Schwark M, Gyorgy A, Vogel T, Akopov S, Mitkovski M, Agoston D, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

