Predicting regional neurodegeneration from the healthy brain functional connectome
- PMID: 22445348
- PMCID: PMC3361461
- DOI: 10.1016/j.neuron.2012.03.004
Predicting regional neurodegeneration from the healthy brain functional connectome
Abstract
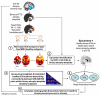
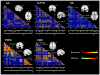
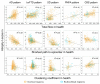
Neurodegenerative diseases target large-scale neural networks. Four competing mechanistic hypotheses have been proposed to explain network-based disease patterning: nodal stress, transneuronal spread, trophic failure, and shared vulnerability. Here, we used task-free fMRI to derive the healthy intrinsic connectivity patterns seeded by brain regions vulnerable to any of five distinct neurodegenerative diseases. These data enabled us to investigate how intrinsic connectivity in health predicts region-by-region vulnerability to disease. For each illness, specific regions emerged as critical network "epicenters" whose normal connectivity profiles most resembled the disease-associated atrophy pattern. Graph theoretical analyses in healthy subjects revealed that regions with higher total connectional flow and, more consistently, shorter functional paths to the epicenters, showed greater disease-related vulnerability. These findings best fit a transneuronal spread model of network-based vulnerability. Molecular pathological approaches may help clarify what makes each epicenter vulnerable to its targeting disease and how toxic protein species travel between networked brain structures.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


Comment in
-
Disintegrating brain networks: from syndromes to molecular nexopathies.Neuron. 2012 Mar 22;73(6):1060-2. doi: 10.1016/j.neuron.2012.03.006. Epub 2012 Mar 21. Neuron. 2012. PMID: 22445334 Free PMC article.
-
Neurodegenerative networking.Nat Rev Neurosci. 2012 Apr 18;13(5):288. doi: 10.1038/nrn3248. Nat Rev Neurosci. 2012. PMID: 22510888 No abstract available.
References
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. - PubMed
-
- Appel SH. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, parkinsonism, and Alzheimer disease. Ann Neurol. 1981;10:499–505. - PubMed
-
- Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Induction of beta (A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Mol Neurobiol. 1994;8:25–39. - PubMed
-
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

