Synthesis and antiviral activity of a series of 1'-substituted 4-aza-7,9-dideazaadenosine C-nucleosides
- PMID: 22446091
- PMCID: PMC7126871
- DOI: 10.1016/j.bmcl.2012.02.105
Synthesis and antiviral activity of a series of 1'-substituted 4-aza-7,9-dideazaadenosine C-nucleosides
Abstract
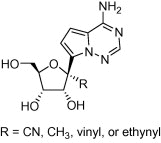
A series of 1'-substituted analogs of 4-aza-7,9-dideazaadenosine C-nucleoside were prepared and evaluated for the potential as antiviral agents. These compounds showed a broad range of inhibitory activity against various RNA viruses. In particular, the whole cell potency against HCV when R=CN was attributed to inhibition of HCV NS5B polymerase and intracellular concentration of the corresponding nucleoside triphosphate.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures



References
-
- Franchetti P., Cappellacci L., Pasqualini M., Petrelli R., Vita P., Jayaram H.N., Horvath Z., Szekeres T., Grifantini M. J. Med. Chem. 2005;48:4983. Sporadic examples of 1′-substituted N-nucleosides prepared for biological evaluation; - PubMed
- Damont A., Dukhan D., Gosselin G., Payronnet J., Storer R. Nucleosides Nucleotides Nucleic Acids. 2007;26:1431. - PubMed
- Yoshimura Y., Kano F., Miyazaki S., Ashida N., Sakada S., Haraguchi K., Itoh Y., Tanaka H., Miyasaka T. Nucleosides Nucleotides Nucleic Acids. 1996;15:305.
-
- Cappellacci P., Barboni G., Palmieri M., Pasqualini M., Grifantini M., Costa B., Martini C., Franchetti P. J. Med. Chem. 2002;45:1196. - PubMed
-
- Olsen D.B., Eldrup A.B., Bartholomew L., Bhat B., Bosserman M.R., Ceccacci A., Colwell L.F., Fay J.F., Flores O.A., Getty K.L., Grobler J.A., LaFemina R.L., Markel E.J., Migliaccino G., Prhavc M., Stahlhut M.W., Tomassini J.E., MacCoss M., Hazuda D.J., Carroll S.S. Antimicrob. Agents Chemother. 2004;48:3944. - PMC - PubMed
-
- Patil S.A., Otter B.A., Klein R.S. Tetrahedron Lett. 1994;35:5339.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

