Research resource: whole-genome estrogen receptor α binding in mouse uterine tissue revealed by ChIP-seq
- PMID: 22446102
- PMCID: PMC3355558
- DOI: 10.1210/me.2011-1311
Research resource: whole-genome estrogen receptor α binding in mouse uterine tissue revealed by ChIP-seq
Abstract
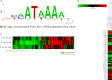
To advance understanding of mechanisms leading to biological and transcriptional endpoints related to estrogen action in the mouse uterus, we have mapped ERα and RNA polymerase II (PolII) binding sites using chromatin immunoprecipitation followed by sequencing of enriched chromatin fragments. In the absence of hormone, 5184 ERα-binding sites were apparent in the vehicle-treated ovariectomized uterine chromatin, whereas 17,240 were seen 1 h after estradiol (E₂) treatment, indicating that some sites are occupied by unliganded ERα, and that ERα binding is increased by E₂. Approximately 15% of the uterine ERα-binding sites were adjacent to (<10 kb) annotated transcription start sites, and many sites are found within genes or are found more than 100 kb distal from mapped genes; however, the density (sites per base pair) of ERα-binding sites is significantly greater adjacent to promoters. An increase in quantity of sites but no significant positional differences were seen between vehicle and E₂-treated samples in the overall locations of ERα-binding sites either distal from, adjacent to, or within genes. Analysis of the PolII data revealed the presence of poised promoter-proximal PolII on some highly up-regulated genes. Additionally, corecruitment of PolII and ERα to some distal enhancer regions was observed. A de novo motif analysis of sequences in the ERα-bound chromatin confirmed that estrogen response elements were significantly enriched. Interestingly, in areas of ERα binding without predicted estrogen response element motifs, homeodomain transcription factor-binding motifs were significantly enriched. The integration of the ERα- and PolII-binding sites from our uterine sequencing of enriched chromatin fragments data with transcriptional responses revealed in our uterine microarrays has the potential to greatly enhance our understanding of mechanisms governing estrogen response in uterine and other estrogen target tissues.
Figures


References
-
- Katzenellenbogen BS, Bhakoo HS, Ferguson ER, Lan NC, Tatee T, Tsai TS, Katzenellenbogen JA. 1979. Estrogen and antiestrogen action in reproductive tissues and tumors. Recent Prog Horm Res 35:259–300 - PubMed
-
- Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. 2003. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol 17:2070–2083 - PubMed
-
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M. 2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

