Novel genital alphapapillomaviruses in baboons (Papio hamadryas anubis) with cervical dysplasia
- PMID: 22446324
- PMCID: PMC3963400
- DOI: 10.1177/0300985812439725
Novel genital alphapapillomaviruses in baboons (Papio hamadryas anubis) with cervical dysplasia
Abstract
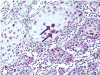
Genital Alphapapillomavirus (αPV) infections are one of the most common sexually transmitted human infections worldwide. Women infected with the highly oncogenic genital human papillomavirus (HPV) types 16 and 18 are at high risk for development of cervical cancer. Related oncogenic αPVs exist in rhesus and cynomolgus macaques. Here the authors identified 3 novel genital αPV types (PhPV1, PhPV2, PhPV3) by PCR in cervical samples from 6 of 15 (40%) wild-caught female Kenyan olive baboons (Papio hamadryas anubis). Eleven baboons had koilocytes in the cervix and vagina. Three baboons had dysplastic proliferative changes consistent with cervical squamous intraepithelial neoplasia (CIN). In 2 baboons with PCR-confirmed PhPV1, 1 had moderate (CIN2, n = 1) and 1 had low-grade (CIN1, n = 1) dysplasia. In 2 baboons with PCR-confirmed PhPV2, 1 had low-grade (CIN1, n = 1) dysplasia and the other had only koilocytes. Two baboons with PCR-confirmed PhPV3 had koilocytes only. PhPV1 and PhPV2 were closely related to oncogenic macaque and human αPVs. These findings suggest that αPV-infected baboons may be useful animal models for the pathogenesis, treatment, and prophylaxis of genital αPV neoplasia. Additionally, this discovery suggests that genital αPVs with oncogenic potential may infect a wider spectrum of non-human primate species than previously thought.
Conflict of interest statement
Figures






References
-
- Burk RD, Ho GYF, Beardsley L, et al. Sexual behavior and partner characteristics are the predominant risk factors for genital human papillomavirus infection in young women. J Infecti Dis. 1996;174:679–689. - PubMed
-
- Campo MS. Animal models of papillomavirus pathogenesis. Virus Res. 2002;89:249–261. - PubMed

