Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'
- PMID: 22446627
- PMCID: PMC3338870
- DOI: 10.1038/nature10975
Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'
Abstract
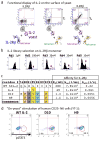
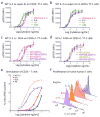
The immunostimulatory cytokine interleukin-2 (IL-2) is a growth factor for a wide range of leukocytes, including T cells and natural killer (NK) cells. Considerable effort has been invested in using IL-2 as a therapeutic agent for a variety of immune disorders ranging from AIDS to cancer. However, adverse effects have limited its use in the clinic. On activated T cells, IL-2 signals through a quaternary 'high affinity' receptor complex consisting of IL-2, IL-2Rα (termed CD25), IL-2Rβ and IL-2Rγ. Naive T cells express only a low density of IL-2Rβ and IL-2Rγ, and are therefore relatively insensitive to IL-2, but acquire sensitivity after CD25 expression, which captures the cytokine and presents it to IL-2Rβ and IL-2Rγ. Here, using in vitro evolution, we eliminated the functional requirement of IL-2 for CD25 expression by engineering an IL-2 'superkine' (also called super-2) with increased binding affinity for IL-2Rβ. Crystal structures of the IL-2 superkine in free and receptor-bound forms showed that the evolved mutations are principally in the core of the cytokine, and molecular dynamics simulations indicated that the evolved mutations stabilized IL-2, reducing the flexibility of a helix in the IL-2Rβ binding site, into an optimized receptor-binding conformation resembling that when bound to CD25. The evolved mutations in the IL-2 superkine recapitulated the functional role of CD25 by eliciting potent phosphorylation of STAT5 and vigorous proliferation of T cells irrespective of CD25 expression. Compared to IL-2, the IL-2 superkine induced superior expansion of cytotoxic T cells, leading to improved antitumour responses in vivo, and elicited proportionally less expansion of T regulatory cells and reduced pulmonary oedema. Collectively, we show that in vitro evolution has mimicked the functional role of CD25 in enhancing IL-2 potency and regulating target cell specificity, which has implications for immunotherapy.
Figures


Comment in
-
Protein engineering: Tighter ties that bind.Nature. 2012 Apr 25;484(7395):463-4. doi: 10.1038/484463a. Nature. 2012. PMID: 22538605 No abstract available.
References
-
- Smith KA. Interleukin-2: inception impact, and implications. Science. 1988;240 (4856):1169–1176. - PubMed
-
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6 (8):595–601. - PubMed
-
- Cosman D, et al. Cloning, sequence and expression of human interleukin-2 receptor. Nature. 1984;312 (5996):768–771. - PubMed
-
- Leonard WJ, et al. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984;311 (5987):626–631. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- T32 GM007365/GM/NIGMS NIH HHS/United States
- AR050942/AR/NIAMS NIH HHS/United States
- U19 AI082719/AI/NIAID NIH HHS/United States
- R01AI51321/AI/NIAID NIH HHS/United States
- R01-GM062868/GM/NIGMS NIH HHS/United States
- R01 GM062868/GM/NIGMS NIH HHS/United States
- R37 AI051321/AI/NIAID NIH HHS/United States
- U19 AI 082719/AI/NIAID NIH HHS/United States
- GM07365/GM/NIGMS NIH HHS/United States
- R01 CA065237/CA/NCI NIH HHS/United States
- T32 AR050942/AR/NIAMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U01 DK078123/DK/NIDDK NIH HHS/United States
- R01 AI051321/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

