Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial
- PMID: 22447964
- PMCID: PMC3400995
- DOI: 10.1164/rccm.201108-1553OC
Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial
Abstract
Rationale: Exacerbations of chronic obstructive pulmonary disease (COPD) and responses to treatment are heterogeneous.
Objectives: Investigate the usefulness of blood eosinophils to direct corticosteroid therapy during exacerbations.
Methods: Subjects with COPD exacerbations were entered into a randomized biomarker-directed double-blind corticosteroid versus standard therapy study. Subjects in the standard arm received prednisolone for 2 weeks, whereas in the biomarker-directed arm, prednisolone or matching placebo was given according to the blood eosinophil count biomarker. Both study groups received antibiotics. Blood eosinophils were measured in the biomarker-directed and standard therapy arms to define biomarker-positive and -negative exacerbations (blood eosinophil count > and ≤ 2%, respectively). The primary outcome was to determine noninferiority in health status using the chronic respiratory questionnaire (CRQ) and in the proportion of exacerbations associated with a treatment failure between subjects allocated to the biomarker-directed and standard therapy arms.
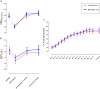
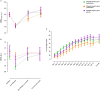
Measurements and main results: There were 86 and 80 exacerbations in the biomarker-directed and standard treatment groups, respectively. In the biomarker-directed group, 49% of the exacerbations were not treated with prednisolone. CRQ improvement after treatment in the standard and biomarker-directed therapy groups was similar (0.8 vs. 1.1; mean difference, 0.3; 95% confidence interval, 0.0-0.6; P = 0.05). There was a greater improvement in CRQ in biomarker-negative exacerbations given placebo compared with those given prednisolone (mean difference, 0.45; 95% confidence interval, 0.01-0.90; P = 0.04). In biomarker-negative exacerbations, treatment failures occurred in 15% given prednisolone and 2% of those given placebo (P = 0.04).
Conclusions: The peripheral blood eosinophil count is a promising biomarker to direct corticosteroid therapy during COPD exacerbations, but larger studies are required.
Figures
References
-
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555 - PubMed
-
- Saetta M, Di SA, Maestrelli P, Turato G, Ruggieri MP, Roggeri A, Calcagni P, Mapp CE, Ciaccia A, Fabbri LM. Airway eosinophilia in chronic bronchitis during exacerbations. Am J Respir Crit Care Med 1994;150:1646–1652 - PubMed
-
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006;173:1114–1121 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

