Molecular mechanisms in the dramatic enhancement of HIV-1 Tat transduction by cationic liposomes
- PMID: 22447980
- PMCID: PMC3382090
- DOI: 10.1096/fj.11-203315
Molecular mechanisms in the dramatic enhancement of HIV-1 Tat transduction by cationic liposomes
Abstract
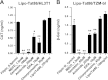
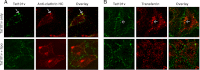
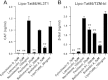
Human immunodeficiency virus type 1 (HIV-1) transactivator of transcription (Tat) protein possesses a unique membrane-transduction property. Interestingly, Tat transduction could be dramatically increased 1000-fold based on LTR-transactivation assay when complexed with cationic liposomes (lipo-Tat), compared with Tat alone. Therefore, underlining mechanisms were explored further. Microscopy and flow cytometry showed that this effect was associated with enhanced membrane binding, large particle formation (1-2 μm) and increased intracellular uptake of Tat fluorescent proteins. Using pharmacological assays and immune colocalizations, it was found that lipid raft-dependent endocytosis and macropinocytosis were major pathways involved in lipo-Tat uptake, and actin-filaments played a major role in intracellular trafficking of lipo-Tat to the nucleus. Furthermore, we found that the Tat hydrophobic domain (aa 36-47) mediated formation of two positively charged molecules into lipo-Tat complexes via hydrophobic bonds, based on LTR-transactivation inhibition assay. Thus, the hydrophobic domain may play an important role in Tat protein uptake and be useful for intracellular delivery of biomacromolecules if coupled together with Tat basic peptide, a cell-penetrating peptide.
Figures





References
-
- Rana T. M., Jeang K. T. (1999) Biochemical and functional interactions between HIV-1 Tat protein and TAR RNA. Arch. Biochem. Biophys. 365, 175–185 - PubMed
-
- Stauber R. H., Pavlakis G. N. (1998) Intracellular trafficking and interactions of the HIV-1 Tat protein. Virology 252, 126–136 - PubMed
-
- Jones K. A., Peterlin B. M. (1994) Control of RNA initiation and elongation at the HIV-1 promoter. Ann. Rev. Biochem. 63, 717–743 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

