Targeting pan-resistant bacteria with antibodies to a broadly conserved surface polysaccharide expressed during infection
- PMID: 22448004
- PMCID: PMC3415848
- DOI: 10.1093/infdis/jis254
Targeting pan-resistant bacteria with antibodies to a broadly conserved surface polysaccharide expressed during infection
Abstract
Background: New therapeutic targets for antibiotic-resistant bacterial pathogens are desperately needed. The bacterial surface polysaccharide poly-β-(1-6)-N-acetyl-glucosamine (PNAG) mediates biofilm formation by some bacterial species, and antibodies to PNAG can confer protective immunity. By analyzing sequenced genomes, we found that potentially multidrug-resistant bacterial species such as Klebsiella pneumoniae, Enterobacter cloacae, Stenotrophomonas maltophilia, and the Burkholderia cepacia complex (BCC) may be able to produce PNAG. Among patients with cystic fibrosis patients, highly antibiotic-resistant bacteria in the BCC have emerged as problematic pathogens, providing an impetus to study the potential of PNAG to be targeted for immunotherapy against pan-resistant bacterial pathogens.
Methods: The presence of PNAG on BCC was assessed using a combination of bacterial genetics, microscopy, and immunochemical approaches. Antibodies to PNAG were tested using opsonophagocytic assays and for protective efficacy against lethal peritonitis in mice.
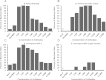
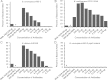
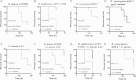
Results: PNAG is expressed in vitro and in vivo by the BCC, and cystic fibrosis patients infected by the BCC species B. dolosa mounted a PNAG-specific opsonophagocytic antibody response. Antisera to PNAG mediated opsonophagocytic killing of BCC and were protective against lethal BCC peritonitis even during coinfection with methicillin-resistant Staphylococcus aureus.
Conclusions: Our findings raise potential new therapeutic options against PNAG-producing bacteria, including even pan-resistant pathogens.
Figures



References
-
- Waters V, Ratjen F. Multidrug-resistant organisms in cystic fibrosis: management and infection-control issues. Expert Rev Anti Infect Ther. 2006;4:807–19. - PubMed
-
- Aaron SD, Vandemheen KL, Ramotar K, et al. Infection with transmissible strains of Pseudomonas aeruginosa and clinical outcomes in adults with cystic fibrosis. JAMA. 2010;304:2145–53. - PubMed
-
- Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–92. - PubMed
-
- Kalish LA, Waltz DA, Dovey M, et al. Impact of Burkholderia dolosa on lung function and survival in cystic fibrosis. Am J Respir Crit Care Med. 2006;173:421–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

