Vesiculation from Pseudomonas aeruginosa under SOS
- PMID: 22448133
- PMCID: PMC3289957
- DOI: 10.1100/2012/402919
Vesiculation from Pseudomonas aeruginosa under SOS
Abstract
Bacterial infections can be aggravated by antibiotic treatment that induces SOS response and vesiculation. This leads to a hypothesis concerning association of SOS with vesiculation. To test it, we conducted multiple analyses of outer membrane vesicles (OMVs) produced from the Pseudomonas aeruginosa wild type in which SOS is induced by ciprofloxacin and from the LexA noncleavable (lexAN) strain in which SOS is repressed. The levels of OMV proteins, lipids, and cytotoxicity increased for both the treated strains, demonstrating vesiculation stimulation by the antibiotic treatment. However, the further increase was suppressed in the lexAN strains, suggesting the SOS involvement. Obviously, the stimulated vesiculation is attributed by both SOS-related and unrelated factors. OMV subproteomic analysis was performed to examine these factors, which reflected the OMV-mediated cytotoxicity and the physiology of the vesiculating cells under treatment and SOS. Thus, SOS plays a role in the vesiculation stimulation that contributes to cytotoxicity.
Figures

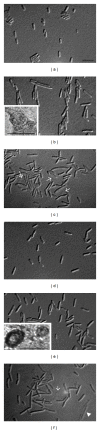
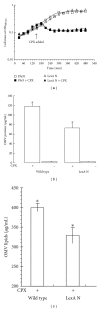
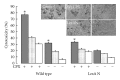
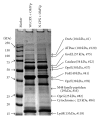
Similar articles
-
Motility of Pseudomonas aeruginosa contributes to SOS-inducible biofilm formation.Res Microbiol. 2013 Dec;164(10):1019-27. doi: 10.1016/j.resmic.2013.10.001. Epub 2013 Oct 11. Res Microbiol. 2013. PMID: 24125694
-
Involvement of the lon protease in the SOS response triggered by ciprofloxacin in Pseudomonas aeruginosa PAO1.Antimicrob Agents Chemother. 2012 Jun;56(6):2879-87. doi: 10.1128/AAC.06014-11. Epub 2012 Mar 26. Antimicrob Agents Chemother. 2012. PMID: 22450976 Free PMC article.
-
Defining the Pseudomonas aeruginosa SOS response and its role in the global response to the antibiotic ciprofloxacin.J Bacteriol. 2006 Oct;188(20):7101-10. doi: 10.1128/JB.00807-06. J Bacteriol. 2006. PMID: 17015649 Free PMC article.
-
Action of quinolones on gene expression and bacterial membranes.Antibiot Chemother (1971). 1987;39:205-14. doi: 10.1159/000414346. Antibiot Chemother (1971). 1987. PMID: 2823690 Review. No abstract available.
-
Mechanisms of ciprofloxacin resistance in Pseudomonas aeruginosa: new approaches to an old problem.J Med Microbiol. 2019 Jan;68(1):1-10. doi: 10.1099/jmm.0.000873. J Med Microbiol. 2019. PMID: 30605076 Review.
Cited by
-
Spontaneous Prophage Induction Contributes to the Production of Membrane Vesicles by the Gram-Positive Bacterium Lacticaseibacillus casei BL23.mBio. 2022 Oct 26;13(5):e0237522. doi: 10.1128/mbio.02375-22. Epub 2022 Oct 6. mBio. 2022. PMID: 36200778 Free PMC article.
-
Extracellular vesicles of mycoplasmas and development of resistance to quinolones in bacteria.Dokl Biochem Biophys. 2014 Jan;454(1):34-7. doi: 10.1134/S1607672914010104. Epub 2014 Mar 16. Dokl Biochem Biophys. 2014. PMID: 24633610 No abstract available.
-
Extracellular Vesicles and Host-Pathogen Interactions: A Review of Inter-Kingdom Signaling by Small Noncoding RNA.Genes (Basel). 2021 Jun 30;12(7):1010. doi: 10.3390/genes12071010. Genes (Basel). 2021. PMID: 34208860 Free PMC article. Review.
-
Bacterial membrane vesicles transport their DNA cargo into host cells.Sci Rep. 2017 Aug 1;7(1):7072. doi: 10.1038/s41598-017-07288-4. Sci Rep. 2017. PMID: 28765539 Free PMC article.
-
Outer Membrane Vesicle Induction and Isolation for Vaccine Development.Front Microbiol. 2021 Feb 4;12:629090. doi: 10.3389/fmicb.2021.629090. eCollection 2021. Front Microbiol. 2021. PMID: 33613498 Free PMC article. Review.
References
-
- Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multidrug-resistant Pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25(10):1353–1364. - PubMed
-
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284(5418):1318–1322. - PubMed
-
- Bielecki P, Glik J, Kawecki M, dos Santos VAPM. Towards understanding Pseudomonas aeruginosa burn wound infections by profiling gene expression. Biotechnology Letters. 2008;30(5):777–790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

