CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE
- PMID: 22448150
- PMCID: PMC3308938
- DOI: 10.1371/journal.pmed.1001194
CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE
Abstract
Background: Most adults infected with HIV achieve viral suppression within a year of starting combination antiretroviral therapy (cART). It is important to understand the risk of AIDS events or death for patients with a suppressed viral load.
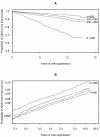
Methods and findings: Using data from the Collaboration of Observational HIV Epidemiological Research Europe (2010 merger), we assessed the risk of a new AIDS-defining event or death in successfully treated patients. We accumulated episodes of viral suppression for each patient while on cART, each episode beginning with the second of two consecutive plasma viral load measurements <50 copies/µl and ending with either a measurement >500 copies/µl, the first of two consecutive measurements between 50-500 copies/µl, cART interruption or administrative censoring. We used stratified multivariate Cox models to estimate the association between time updated CD4 cell count and a new AIDS event or death or death alone. 75,336 patients contributed 104,265 suppression episodes and were suppressed while on cART for a median 2.7 years. The mortality rate was 4.8 per 1,000 years of viral suppression. A higher CD4 cell count was always associated with a reduced risk of a new AIDS event or death; with a hazard ratio per 100 cells/µl (95% CI) of: 0.35 (0.30-0.40) for counts <200 cells/µl, 0.81 (0.71-0.92) for counts 200 to <350 cells/µl, 0.74 (0.66-0.83) for counts 350 to <500 cells/µl, and 0.96 (0.92-0.99) for counts ≥500 cells/µl. A higher CD4 cell count became even more beneficial over time for patients with CD4 cell counts <200 cells/µl.
Conclusions: Despite the low mortality rate, the risk of a new AIDS event or death follows a CD4 cell count gradient in patients with viral suppression. A higher CD4 cell count was associated with the greatest benefit for patients with a CD4 cell count <200 cells/µl but still some slight benefit for those with a CD4 cell count ≥500 cells/µl.
Conflict of interest statement
HCB has received travel grants, honoraria and unrestricted research grants from various pharmaceutical companies, including GlaxoSmithKline (GSK), Bristol-Myers Squibb (BMS), Gilead, Janssen, Roche, Abbott, Tibotec, Boehringer-Ingelheim, and ViiV Healthcare; HF has received payment for participation on advisory boards, unrestricted educational grants or travel grants from Abbott, BMS, ViiV Healthcare, Roche, Gilead, MSD, Boehringer-Ingelheim, and Tibotec-Janssen, and unrestricted research support from Gilead, MSD, and Roche; FG has received honoraria for participation on advisory boards, unrestricted educational grants or travel grants from Abbott, BMS, ViiV Healthcare, Gilead, MSD, Boehringer-Ingelheim, and Tibotec-Janssen; GC has received honoraria from Roche; PR has served as a scientific advisor to Boehringer Ingelheim Pharmaceuticals, BMS, Gilead Sciences, GSK, ViiV Healthcare, Merck, Theratechnologies, Tibotec Therapeutics, Tobira Therapeutics, and Ferrer, has served on data and safety monitoring boards and endpoint adjudication committees for Tibotec Therapeutics, has received honoraria for speaking engagements at scientific conferences from Boehringer Ingelheim Pharmaceuticals, BMS, Gilead Sciences, GSK, and Theratechnologies, has received research support from Gilead Sciences, ViiV Healthcare, Tibotec, BMS, Merck, Abbott, and Boehringer Ingelheim Pharmaceuticals; JMM has received payment for participation on advisory boards, consultancy or lecturing from Abbott, Boehringer-Ingelheim, BMS, Cubist, GSK, Gilead Sciences, Janssen-Cilag, Merck, Novartis, Pfizer, Roche, Schering-Plough, Theravance, and ViiV. JY, MP, LM, SA, SG, FR, BG, MS, NO, OK, AC, SDW, JM, JK, CC, and JG all declare no conflicts of interest.
Figures
References
-
- May MT, Sterne JA, Costagliola D, Sabin CA, Phillips AN, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451–458. - PubMed
-
- Vo TT, Ledergerber B, Keiser O, Hirschel B, Furrer H, et al. Durability and outcome of initial antiretroviral treatments received during 2000–2005 by patients in the Swiss HIV Cohort Study. J Infect Dis. 2008;197:1685–1694. - PubMed
-
- Egger M, May M, Chene G, Phillips AN, Ledergerber B, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. - PubMed
-
- Chene G, Sterne JA, May M, Costagliola D, Ledergerber B, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies. Lancet. 2003;362:679–686. - PubMed
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2011. Available: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 17 October 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

